Deck 3: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/72
Play
Full screen (f)
Deck 3: Acids and Bases
1
Is the indicated compound acting an acid or a base in the following reaction? 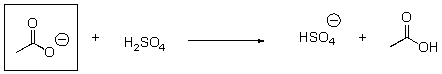
A)Acid
B)Base
C)Neither

A)Acid
B)Base
C)Neither
Base
2
What is an ionic reaction?
It is a reaction in which ions participate as the reactants.
3
Identify the acid and the base and draw the mechanism. 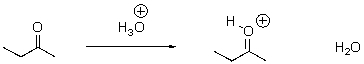

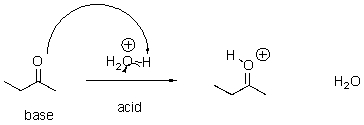
4
Is the indicated compound acting an acid or a base in the following reaction? 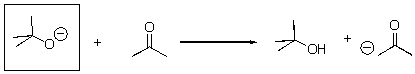
A)Acid
B)Base
C)Neither
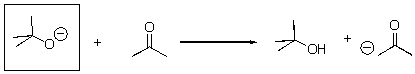
A)Acid
B)Base
C)Neither

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
5
What is wrong with the following arrow? Please draw the correct mechanism. 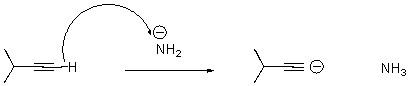
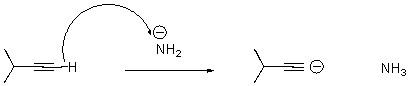

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
6
Identify the acid and the base and draw the mechanism. 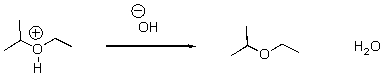
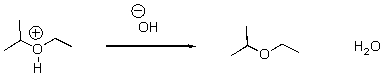

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
7
Identify the acid and the base and draw the mechanism. 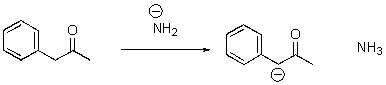


Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
8
Is the indicated compound acting an acid or a base in the following reaction? 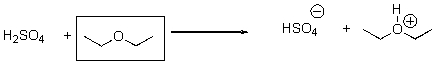
A)Acid
B)Base
C)Neither

A)Acid
B)Base
C)Neither

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
9
What is the conjugate base of the following acid? 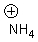


Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
10
Identify the acid and the base and draw the mechanism. 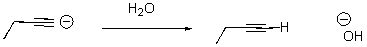


Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
11
Which side will the following acid-base reaction favor? 
A)The right
B)The left
C)Neither

A)The right
B)The left
C)Neither

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
12
What is the conjugate base of the following acid? 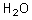
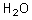

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
13
Is the indicated compound acting an acid or a base in the following reaction? 
A)Acid
B)Base
C)Neither

A)Acid
B)Base
C)Neither

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
14
What is wrong with the following arrow? 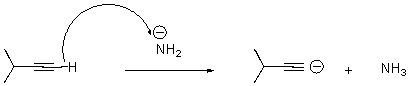
A)It should start on the alkyne carbon.
B)It should start on a hydrogen attached to the nitrogen.
C)It should start on the anion on nitrogen,end at the H on the alkyne,and a second arrow should start at the bond between the C and H on the alkyne and end on the terminal carbon of the alkyne.
D)There should be two arrows - one from nitrogen and one from the alkyne carbon.
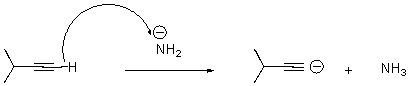
A)It should start on the alkyne carbon.
B)It should start on a hydrogen attached to the nitrogen.
C)It should start on the anion on nitrogen,end at the H on the alkyne,and a second arrow should start at the bond between the C and H on the alkyne and end on the terminal carbon of the alkyne.
D)There should be two arrows - one from nitrogen and one from the alkyne carbon.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
15
In the following reaction,identify the acid and base as well as the conjugate acid and base. 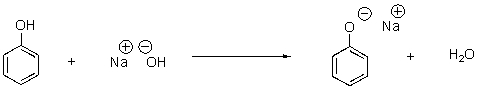


Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
16
Is the indicated compound acting an acid or a base in the following reaction? 
A)Acid
B)Base
C)Neither

A)Acid
B)Base
C)Neither

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
17
Draw arrows to indicate the movement of electrons in the following reaction. 


Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
18
Draw arrows to indicate the movement of electrons in the following reaction. 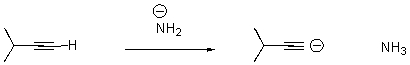
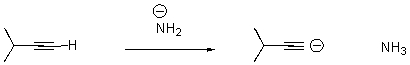

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
19
Provide a definition of a Bronsted-Lowry acid.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
20
Identify the acid and the base and draw the mechanism. 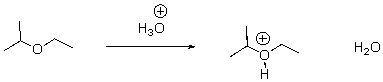


Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
21
Predict the position of equilibrium for the following reaction. 
A)To the left
B)To the right
C)No reaction

A)To the left
B)To the right
C)No reaction

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
22
Provide the correct Keq for the following reaction. 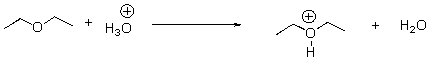


Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
23
Which side will the following acid-base reaction favor? 
A)The right
B)The left
C)Neither

A)The right
B)The left
C)Neither

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
24
What are two factors that influence the acidity of a compound?

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
25
Predict the position of equilibrium for the following reaction. 
A)To the left
B)To the right
C)No reaction

A)To the left
B)To the right
C)No reaction

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
26
Predict the position of equilibrium for the following reaction. 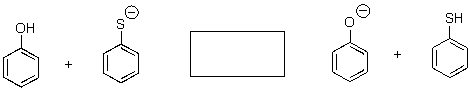
A)To the left
B)To the right
C)No reaction

A)To the left
B)To the right
C)No reaction

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
27
Which side will the following acid-base reaction favor? 
A)The right
B)The left
C)Neither

A)The right
B)The left
C)Neither

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
28
Why is acetic acid more acidic than ethanol when the acidic proton in both cases is attached to oxygen?

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is the strongest acid?
A)HF
B)HBr
C)HCl
D)HI
A)HF
B)HBr
C)HCl
D)HI

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
30
Which side will the following acid-base reaction favor? 
A)The right
B)The left
C)Neither

A)The right
B)The left
C)Neither

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
31
Which side will the following acid-base reaction favor? 
A)The right
B)The left
C)Neither

A)The right
B)The left
C)Neither

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
32
Which side will the following acid-base reaction favor? 
A)The right
B)The left
C)Neither

A)The right
B)The left
C)Neither

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
33
Identify the most acidic proton on the following compound. 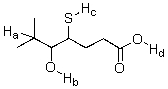
A)Ha
B)Hb
C)Hc
D)Hd

A)Ha
B)Hb
C)Hc
D)Hd

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
34
Which side will the following acid-base reaction favor? 
A)The right
B)The left
C)Neither

A)The right
B)The left
C)Neither

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
35
Could water protonate the following compound? 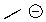
A)Yes
B)No
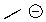
A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
36
What is the difference between Ka and pKa?

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
37
Predict the position of equilibrium for the following reaction. 
A)To the left
B)To the right
C)No reaction

A)To the left
B)To the right
C)No reaction

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
38
Identify the most acidic proton on the following compound. 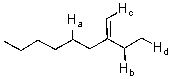
A)Ha
B)Hb
C)Hc
D)Hd

A)Ha
B)Hb
C)Hc
D)Hd

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is the strongest acid?
A)HOMe
B)HSMe
C)HSeMe
D)HTeMe
A)HOMe
B)HSMe
C)HSeMe
D)HTeMe

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
40
Could water protonate the following compound? 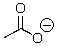
A)Yes
B)No

A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
41
Could water protonate the following compound? HOSO3-
A)Yes
B)No
A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
42
Can this base exist in water? 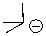
A)Yes
B)No
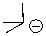
A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
43
What is the counterion to methoxide in the following compound? NaOMe
A)Na+
B)O
C)Me
D)OMe
A)Na+
B)O
C)Me
D)OMe

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
44
Could ammonia (NH3)deprotonate CH4?
A)Yes
B)No
A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
45
What is a cation?

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
46
Could the amide anion (- NH2)deprotonate CH4?
A)Yes
B)No
A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
47
What is the strongest base that can exist in water?

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
48
Can this base exist in water? 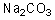
A)Yes
B)No
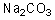
A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
49
Can this base exist in water? 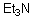
A)Yes
B)No
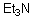
A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
50
Can this base exist in water? 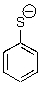
A)Yes
B)No

A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
51
Can this base exist in hexane? 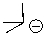
A)Yes
B)No
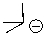
A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
52
Can this base exist in water? 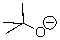
A)Yes
B)No

A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
53
Could water protonate the following compound? 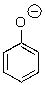
A)Yes
B)No

A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
54
Could hydroxide deprotonate CH4?
A)Yes
B)No
A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
55
Could the amide anion (- NH2)deprotonate the following compound? 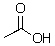
A)Yes
B)No

A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
56
What is the counterion of iodide in the following compound? AgI
A)I
B)Ag+
C)gI
D)AI
A)I
B)Ag+
C)gI
D)AI

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
57
Can this base exist in liquid ammonia? 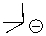
A)Yes
B)No
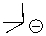
A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
58
Explain the leveling effect.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
59
Could ammonia (NH3)deprotonate the following compound? 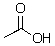
A)Yes
B)No

A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
60
Could hydroxide deprotonate the following compound? 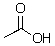
A)Yes
B)No

A)Yes
B)No

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
61
Which is the best descriptor for the following compound? 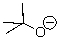
A)Bronsted-Lowry Acid
B)Bronsted-Lowry Base
C)Lewis Acid
D)Lewis Base

A)Bronsted-Lowry Acid
B)Bronsted-Lowry Base
C)Lewis Acid
D)Lewis Base

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
62
Into which of the following categories does the following compound belong? 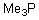
A)Bronsted-Lowry Acid
B)Bronsted-Lowry Base
C)Lewis Acid
D)Lewis Base
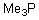
A)Bronsted-Lowry Acid
B)Bronsted-Lowry Base
C)Lewis Acid
D)Lewis Base

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
63
What is the counterion of the t-butyl carbanion in the following compound? 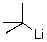
A)tert-butyl
B)C
C)Li+
D)H

A)tert-butyl
B)C
C)Li+
D)H

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
64
Into which of the following categories does the following compound belong? 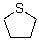
A)Bronsted-Lowry Acid
B)Bronsted-Lowry Base
C)Lewis Acid
D)Lewis Base

A)Bronsted-Lowry Acid
B)Bronsted-Lowry Base
C)Lewis Acid
D)Lewis Base

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
65
Into which of the following categories does the following compound belong? 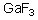
A)Bronsted-Lowry Acid
B)Bronsted-Lowry Base
C)Lewis Acid
D)Lewis Base
A)Bronsted-Lowry Acid
B)Bronsted-Lowry Base
C)Lewis Acid
D)Lewis Base

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
66
For the following reaction,draw the mechanism. 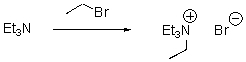


Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
67
In the following reaction,identify the Lewis acid and the Lewis base. 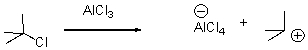
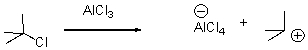

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
68
Into which of the following categories does the following compound belong? 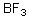
A)Bronsted-Lowry Acid
B)Bronsted-Lowry Base
C)Lewis Acid
D)Lewis Base
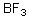
A)Bronsted-Lowry Acid
B)Bronsted-Lowry Base
C)Lewis Acid
D)Lewis Base

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
69
For the following reaction,draw the mechanism. 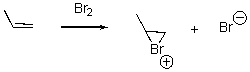


Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
70
For the following reaction,draw the mechanism. 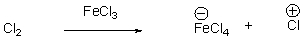
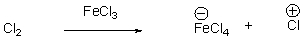

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
71
What is the counterion of t-butoxide in the following compound? 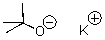
A)K+
B)O
C)tert-butoxide
D)tert-butyl
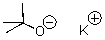
A)K+
B)O
C)tert-butoxide
D)tert-butyl

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
72
Into which of the following categories does the following compound belong? 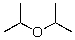
A)Bronsted-Lowry Acid
B)Bronsted-Lowry Base
C)Lewis Acid
D)Lewis Base

A)Bronsted-Lowry Acid
B)Bronsted-Lowry Base
C)Lewis Acid
D)Lewis Base

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck


