Exam 3: Acids and Bases
Exam 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties86 Questions
Exam 2: Molecular Representations114 Questions
Exam 3: Acids and Bases72 Questions
Exam 4: Alkanes and Cycloalkanes66 Questions
Exam 5: Stereoisomerism94 Questions
Exam 6: Chemical Reactivity and Mechanisms86 Questions
Exam 7: Substitution Reactions103 Questions
Exam 8: Alkenes: Structure and Preparation Via Elimination Reactions76 Questions
Exam 9: Addition Reactions of Alkenes40 Questions
Exam 10: Alkynes161 Questions
Exam 11: Radical Reactions52 Questions
Exam 12: Synthesis55 Questions
Exam 13: Alcohols and Phenols71 Questions
Exam 14: Ethers and Epoxides; Thiols and Sulfides102 Questions
Exam 16: Nuclear Magnetic Resonance Spectroscopy90 Questions
Exam 15: Infrared Spectroscopy and Mass Spectrometry108 Questions
Exam 17: Conjugated Pi Systems and Pericyclic Reactions45 Questions
Exam 18: Aromatic Compounds79 Questions
Exam 19: Aromatic Substitution Reactions64 Questions
Exam 20: Aldehydes and Ketones97 Questions
Exam 21: Carboxylic Acids and Their Derivatives58 Questions
Exam 22: Alpha Carbon Chemistry: Enols and Enolates89 Questions
Exam 23: Amines65 Questions
Exam 24: Carbohydrates101 Questions
Exam 25: Amino Acids, Peptides, and Proteins99 Questions
Exam 26: Lipids82 Questions
Exam 27: Synthetic Polymers85 Questions
Select questions type
In the following reaction,identify the Lewis acid and the Lewis base. 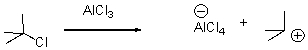
Free
(Essay)
4.8/5  (33)
(33)
Correct Answer:
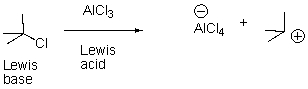
Which side will the following acid-base reaction favor? 
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
A
What is the difference between Ka and pKa?
Free
(Essay)
4.8/5  (37)
(37)
Correct Answer:
pKa = -logKa
Draw arrows to indicate the movement of electrons in the following reaction. 
(Essay)
4.9/5  (32)
(32)
What is the counterion of the t-butyl carbanion in the following compound? 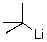
(Multiple Choice)
4.8/5  (29)
(29)
Is the indicated compound acting an acid or a base in the following reaction? 
(Multiple Choice)
4.9/5  (45)
(45)
Into which of the following categories does the following compound belong? 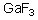
(Multiple Choice)
4.8/5  (37)
(37)
Predict the position of equilibrium for the following reaction. 
(Multiple Choice)
4.8/5  (37)
(37)
Predict the position of equilibrium for the following reaction. 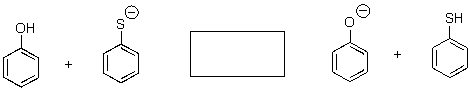
(Multiple Choice)
4.8/5  (34)
(34)
In the following reaction,identify the acid and base as well as the conjugate acid and base. 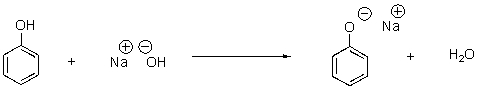
(Essay)
5.0/5  (42)
(42)
Predict the position of equilibrium for the following reaction. 
(Multiple Choice)
5.0/5  (36)
(36)
Draw arrows to indicate the movement of electrons in the following reaction. 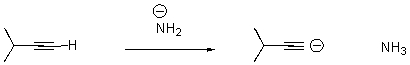
(Essay)
4.9/5  (38)
(38)
Showing 1 - 20 of 72
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)