Exam 1: Structure and Bonding in Organic Molecules
Exam 1: Structure and Bonding in Organic Molecules50 Questions
Exam 2: Structure and Reactivity: Acids and Bases,polar and Nonpolar Molecules39 Questions
Exam 3: Reactions of Alkanes: Bond-Dissociation Energies,radical Halogenation,and Relative Reactivity34 Questions
Exam 4: Cycloalkanes30 Questions
Exam 5: Stereoisomers44 Questions
Exam 6: Properties and Reactions of Haloalkanes: Bimolecular Nucleophilic Substitution38 Questions
Exam 7: Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination25 Questions
Exam 8: Hydroxy of Functional Group: Alcohols: Properties,preparation,and Strategy of Synthesis39 Questions
Exam 9: Further Reactions of Alcohols and the Chemistry of Ethers46 Questions
Exam 10: Using Nuclear Magnetic Resonance Spectroscopy to Deduce Structure43 Questions
Exam 11: Alkenes: Infrared Spectroscopy and Mass Spectrometry47 Questions
Exam 12: Reactions to Alkenes44 Questions
Exam 13: Alkynes: the Carbon27 Questions
Exam 14: Delocalized Pi Systems: Investigation by Ultraviolet and Visible Spectroscopy Interlude34 Questions
Exam 15: Benzene and Aromaticity: Electrophilic Aromatic Substitution29 Questions
Exam 16: Electrophilic Attack on Derivatives of Benzene: Substituents Control Regioselectivity30 Questions
Exam 17: Aldehydes and Ketones: the Carbonyl Group32 Questions
Exam 18: Enols,enolates,and the Aldol Condensation: A,b-Unsaturated Aldehydes and Ketones31 Questions
Exam 19: Carboxylic Acids27 Questions
Exam 20: Carboxylic Acid Derivatives44 Questions
Exam 21: Amines and Their Derivatives: Functional Groups Containing Nitrogen20 Questions
Exam 22: Chemistry of the Benzene Substituents: Alkylbenzenes,phenols,and Benzenamines32 Questions
Exam 23: Ester Enolates and the Claisen Condensation: Synthesis of B-Dicarbonyl Compounds; Acyl Anion Equivalents29 Questions
Exam 24: Carbohydrates: Polyfunctional Compounds in Nature27 Questions
Exam 25: Heterocycles: Heteroatoms in Cyclic Organic Compounds23 Questions
Exam 26: Amino Acids,peptides,proteins,and Nucleic Acids: Nitrogen-Containing Polymers in Nature39 Questions
Select questions type
Which of the following statements are true of sp orbitals?
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
E
What is the hybridization of the each of the labeled atoms for the potent neurotoxin (-)-gephyrotoxin? 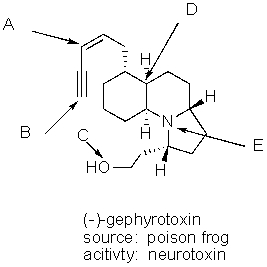
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
D
Which of the following represent resonance contributing Lewis structures for CH2N2?
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
E
A hydrocarbon with a double bond and a ring will have the general formula?
(Multiple Choice)
4.9/5  (35)
(35)
The nitrogen of trimethylamine [(CH3)3N] contains how many lone pairs of electrons?
(Multiple Choice)
4.7/5  (37)
(37)
In the following molecule,how many carbon atoms are in the sp3 hybridization state? 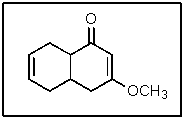
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following molecules are most likely to be held together by a purely covalent bond?
(Multiple Choice)
4.9/5  (36)
(36)
The lone-pair of electrons on nitrogen in the following molecule reside in what type of orbital? 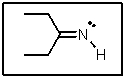
(Multiple Choice)
4.9/5  (25)
(25)
Of those indicated,which would be the shortest carbon-carbon bond in -cadinene? 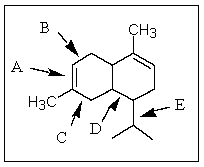
(Multiple Choice)
4.9/5  (32)
(32)
Camptothecin is an important anticancer compound; how many carbons are in the sp hybridization state? 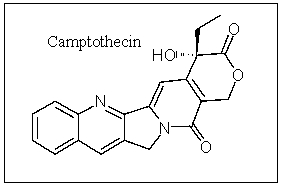
(Multiple Choice)
4.8/5  (34)
(34)
In the following molecule,how many carbon atoms are in the sp2 hybridization state? 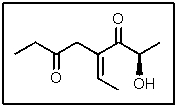
(Multiple Choice)
4.8/5  (43)
(43)
In the following molecule,how many carbon atoms are in the sp hybridization state? 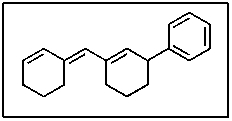
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following pairs are not resonance structures of each other?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following most correctly defines "structural isomers"?
(Multiple Choice)
4.8/5  (38)
(38)
What is the molecular formula of carvone,the major volatile compound in caraway oil? 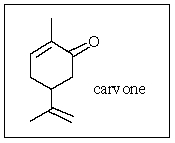
(Multiple Choice)
4.8/5  (44)
(44)
In the following molecule,how many carbon atoms are in the sp3 hybridization state? 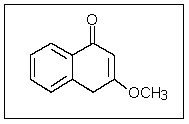
(Multiple Choice)
4.9/5  (34)
(34)
How many sp2 hybridized carbon atoms are in the potent anticancer compound hydroxymethylacylfulvene? 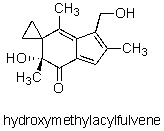
(Multiple Choice)
4.7/5  (28)
(28)
Showing 1 - 20 of 50
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)