Exam 6: Understanding Organic Reactions
Exam 1: Structure and Bonding77 Questions
Exam 2: Acids and Bases59 Questions
Exam 3: Introduction to Organic Molecules and Functional Groups56 Questions
Exam 4: Alkanes64 Questions
Exam 5: Stereochemistry76 Questions
Exam 6: Understanding Organic Reactions53 Questions
Exam 7: Alkyl Halides and Nucleophilic Substitution73 Questions
Exam 8: Alkyl Halides and Elimination Reactions52 Questions
Exam 9: Alcohols,ethers,and Related Compounds60 Questions
Exam 10: Alkenes and Addition Reactions54 Questions
Exam 11: Alkynes and Synthesis51 Questions
Exam 12: Oxidation and Reduction51 Questions
Exam 13: Radical Reactions51 Questions
Exam 14: Conjugation,resonance,and Dienes53 Questions
Exam 15: Benzene and Aromatic Compounds47 Questions
Exam 16: Reactions of Aromatic Compounds60 Questions
Exam 17: Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation and Reduction59 Questions
Exam 18: Aldehydes and Ketones - Nucleophilic Addition52 Questions
Exam 19: Carboxylic Acids and the Acidity of the O-H Bond53 Questions
Exam 20: Carboxylic Acids and Their Derivatives Nucleophilic Acyl Substitution50 Questions
Exam 21: Substitution Reactions of Carbonyl Compounds at the a Carbon47 Questions
Exam 22: Carbonyl Condensation Reactions51 Questions
Exam 23: Amines65 Questions
Exam 24: Carbon-Carbon Bond-Forming Reactions in Organic Synthesis47 Questions
Exam 25: Pericyclic Reactions62 Questions
Exam 26: Carbohydrates50 Questions
Exam 27: Amino Acids and Proteins46 Questions
Exam 28: Synthetic Polymers45 Questions
Exam 29: Lipids45 Questions
Exam 30: Spectroscopy A: Mass Spectrometry19 Questions
Exam 31: Spectroscopy B: Infrared Spectroscopy39 Questions
Exam 32: Spectroscopy C: Nuclear Magnetic Resonance Spectroscopy51 Questions
Select questions type
What type of bond cleavage takes place in/what type of intermediate is produced in the following reaction? 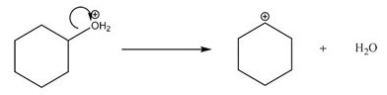
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
C
For which of the following reactions is ΔS° a positive value? 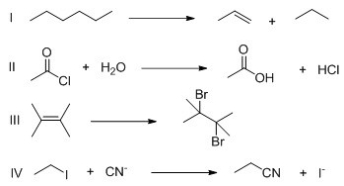
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
A
Which of the following statements about enzymes is true?
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
D
Which of the following statements about bond breaking is not true?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following letters represents DH° for the forward reaction in the following energy diagram? 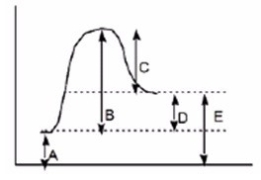
(Multiple Choice)
4.9/5  (31)
(31)
The equilibrium constant for the conversion of A to D is predicted to be which of the following? 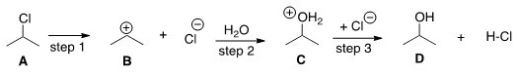
(Multiple Choice)
4.8/5  (36)
(36)
What type of reaction does the following conversion represent? 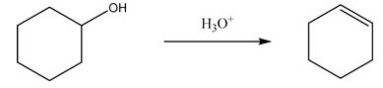
(Multiple Choice)
4.8/5  (37)
(37)
How many transition states and intermediates would the reaction profile have for the reaction shown below? 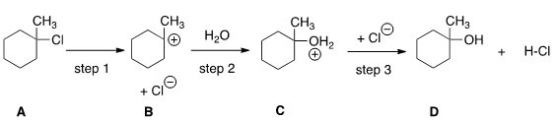
(Multiple Choice)
4.8/5  (33)
(33)
A decrease in which of the following results in an increase in the rate of a chemical reaction?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following statements about a catalyst is true?
(Multiple Choice)
4.8/5  (40)
(40)
If the conversion of A to B is slow and B to C is fast,what is the rate equation for this reaction? 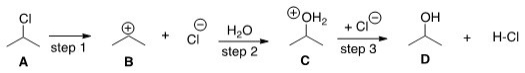
(Multiple Choice)
4.7/5  (35)
(35)
Which of the following statements about bond breaking is true?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following statements about substitution reactions is true?
(Multiple Choice)
4.8/5  (35)
(35)
The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism: 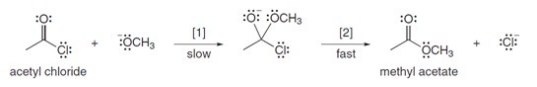 If the concentrations of both -OCH3 and acetyl chloride were increased 2 times,what would happen to the rate of the reaction?
If the concentrations of both -OCH3 and acetyl chloride were increased 2 times,what would happen to the rate of the reaction?
(Multiple Choice)
4.9/5  (37)
(37)
What kind of reaction does the conversion of A to B represent? 
(Multiple Choice)
4.7/5  (27)
(27)
Which of the following statements about equilibrium is true?
(Multiple Choice)
4.9/5  (36)
(36)
Using the bond dissociation energies given,calculate DH° for the following reaction. 
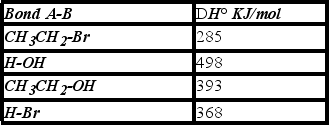
(Multiple Choice)
4.9/5  (39)
(39)
Showing 1 - 20 of 53
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)