Exam 24: Carbon: Not Just Another Element
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: the Tools of Quantitative Chemistry73 Questions
Exam 3: Atoms, Molecules, and Ions104 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions69 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure93 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals66 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids64 Questions
Exam 13: The Solid State67 Questions
Exam 14: Solutions and Their Behavior80 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions74 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria75 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases97 Questions
Exam 18: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria87 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy70 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions83 Questions
Exam 21: Environmental Chemistry: Earths Environment, Energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements81 Questions
Exam 23: The Chemistry of the Transition Elements80 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry40 Questions
Exam 26: Nuclear Chemistry189 Questions
Select questions type
What is the balanced chemical equation for the combustion of 4-methyl-1-pentene?
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
D
The functional group RCONR' is characteristic of a(n) ____.
Free
(Multiple Choice)
4.9/5  (28)
(28)
Correct Answer:
C
What is the major product upon addition of HBr to 1-hexene?
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
C
When sulfur is added to rubber and the mixture is heated, the resulting rubber is still elastic but much stronger. This process is called
(Multiple Choice)
4.9/5  (31)
(31)
Which of the structures below has the common name p-dichlorobenzene (where p- is para)?
(Multiple Choice)
4.9/5  (40)
(40)
Which one of the following hydrocarbons has cis and trans isomers?
(Multiple Choice)
4.7/5  (33)
(33)
How many structures (i.e., unique isomers) exist for tribromobenzene?
(Multiple Choice)
4.8/5  (46)
(46)
Which of the following structural formulas contains an error?
(Multiple Choice)
4.9/5  (40)
(40)
Molecules with nonsuperimposable mirror images are termed chiral. Pairs of nonsuperimposable, mirror image molecules are called ________.
(Short Answer)
4.9/5  (37)
(37)
Which of the following chemical equations depicts a halogenation reaction with benzene?
(Multiple Choice)
4.7/5  (37)
(37)
The process by which long chain hydrocarbons in petroleum are shortened is called ________.
(Short Answer)
4.8/5  (37)
(37)
Low density polyethylene (LDPE) is most likely to be used in
(Multiple Choice)
4.8/5  (35)
(35)
The carbon-carbon bonding of cyclopropane is shown below. How many hydrogen atoms are needed to complete the structural formula of cyclopropane?
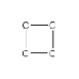
(Multiple Choice)
4.8/5  (29)
(29)
Showing 1 - 20 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)