Exam 24: Carbon: Not Just Another Element
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: the Tools of Quantitative Chemistry73 Questions
Exam 3: Atoms, Molecules, and Ions104 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions69 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure93 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals66 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids64 Questions
Exam 13: The Solid State67 Questions
Exam 14: Solutions and Their Behavior80 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions74 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria75 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases97 Questions
Exam 18: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria87 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy70 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions83 Questions
Exam 21: Environmental Chemistry: Earths Environment, Energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements81 Questions
Exam 23: The Chemistry of the Transition Elements80 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry40 Questions
Exam 26: Nuclear Chemistry189 Questions
Select questions type
Which of the following functional groups does not contain an oxygen atom?
(Multiple Choice)
4.8/5  (40)
(40)
Putrescine and cadaverine are two compounds associated with decaying flesh or bad breath. These compounds belong to a class of molecules called ____.
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following is an example of a hydrocarbon structural formula?
(Multiple Choice)
4.9/5  (40)
(40)
Which one of the following statements concerning isomers is INCORRECT?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following is a geometric isomer of the structure given below? 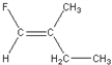
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following class of compounds is responsible for many of the distinctive odors of artificial flavors and perfumes?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following alkenes is capable of forming cis-trans isomers?
(Multiple Choice)
4.7/5  (34)
(34)
Cis-1,2-dichloroethylene and trans-1,2-dichloroethylene are examples of _____ isomers.
(Short Answer)
4.8/5  (28)
(28)
The N atom in an amide has _____ and bond angles of about _____.
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following alcohols is also called rubbing alcohol?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following chemical equations depicts an alkylation reaction?
(Multiple Choice)
4.8/5  (41)
(41)
Formulas for derivatives of hydrocarbons may be written as R−X, where R is a hydrocarbon lacking a hydrogen atom and X is a functional group. Which of the following formulas represents an ether?
(Multiple Choice)
4.7/5  (44)
(44)
Formulas for derivatives of hydrocarbons may be written as R−X, where R is a hydrocarbon lacking a hydrogen atom and X is a functional group. Which of the following formulas represents a secondary amine?
(Multiple Choice)
4.7/5  (34)
(34)
What is the name of the product of the reaction that occurs when a mixture of methanol and propionic acid is heated in the presence of acid?
(Multiple Choice)
4.7/5  (43)
(43)
Showing 61 - 80 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)