Exam 23: The Chemistry of the Transition Elements
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: the Tools of Quantitative Chemistry73 Questions
Exam 3: Atoms, Molecules, and Ions104 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions69 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure93 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals66 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids64 Questions
Exam 13: The Solid State67 Questions
Exam 14: Solutions and Their Behavior80 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions74 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria75 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases97 Questions
Exam 18: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria87 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy70 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions83 Questions
Exam 21: Environmental Chemistry: Earths Environment, Energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements81 Questions
Exam 23: The Chemistry of the Transition Elements80 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry40 Questions
Exam 26: Nuclear Chemistry189 Questions
Select questions type
All of the following metals are generally found in nature as either oxides or sulfides, EXCEPT ____.
(Multiple Choice)
4.9/5  (39)
(39)
The spectrochemical series is CN- > NO2- > en > NH3 > H2O > OH- > F- >Cl- > Br- >I-
Which of the following complexes will absorb visible light of the shortest wavelength?
(Multiple Choice)
5.0/5  (41)
(41)
Which of the following coordination compounds will immediately form a precipitate when combined with an AgNO3 solution?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following ions is responsible for the red color of ruby (gemstone)?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following ligands is responsible for the largest d orbital splitting?
(Multiple Choice)
4.9/5  (40)
(40)
Below is a list of complex ions and their structures. Which of the following pairs is incorrectly matched? 
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following d-block ion electron configurations may be high spin or low spin when placed in an octahedral ligand field?
(Multiple Choice)
4.9/5  (42)
(42)
As bound ligands, which of the following causes the largest splitting of d-orbitals?
(Multiple Choice)
4.8/5  (36)
(36)
All of the following molecules or ions can act as polydentate ligands EXCEPT ____.
(Multiple Choice)
4.9/5  (42)
(42)
If an element can be obtained profitably from a mineral, the mineral is called a(n) _____.
(Multiple Choice)
4.7/5  (40)
(40)
If an octahedral Fe+2 complex is diamagnetic, which of the following sets of conditions best describes the complex?
(Multiple Choice)
4.9/5  (41)
(41)
How many donor atoms are present in the ligand shown below? (Not all lone pairs of electrons are explicitly shown.) 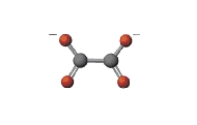
(Multiple Choice)
4.9/5  (36)
(36)
The iron produced in a blast furnace contains significant impurities of carbon, phosphorus, and sulfur. These impurities are removed in a basic oxygen furnace, which converts the impurities to ________.
(Multiple Choice)
4.9/5  (35)
(35)
Ores are often found mixed with impurities, such as sand and clay. In metallurgy, these impurities are called _____.
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following sets of elements or compounds are used to reduce iron(III) oxide (Fe2O3) to impure iron in a blast furnace?
(Multiple Choice)
4.8/5  (38)
(38)
Which element from the first transition series does NOT react with hydrochloric acid?
(Multiple Choice)
4.9/5  (43)
(43)
The flexibility, strength, hardness, and malleability of carbon steel is affected by a heating and cooling process called ____.
(Multiple Choice)
4.8/5  (32)
(32)
The calcium silicate formed in the blast furnace reduction of iron ore is called ____.
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following pairs of coordination compounds are coordination isomers?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 21 - 40 of 80
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)