Exam 9: Bonding and Molecular Structure
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: the Tools of Quantitative Chemistry73 Questions
Exam 3: Atoms, Molecules, and Ions104 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions69 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure93 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals66 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids64 Questions
Exam 13: The Solid State67 Questions
Exam 14: Solutions and Their Behavior80 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions74 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria75 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases97 Questions
Exam 18: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria87 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy70 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions83 Questions
Exam 21: Environmental Chemistry: Earths Environment, Energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements81 Questions
Exam 23: The Chemistry of the Transition Elements80 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry40 Questions
Exam 26: Nuclear Chemistry189 Questions
Select questions type
If a molecule has a positive and negative end, the molecule is said to have a(n) ________ moment.
(Short Answer)
4.8/5  (43)
(43)
What is the formal charge on each atom in dichloromethane, CH2Cl2?
(Multiple Choice)
4.9/5  (34)
(34)
What is the electron-pair geometry around an atom in a molecule or ion which is surrounded by two lone pairs of electrons and three single bonds.
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following is the correct Lewis dash formula for carbon diselenide?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following molecules or ions are likely to be free radicals: N2O, NO, and NO2?
(Multiple Choice)
4.7/5  (39)
(39)
Rank the following covalent bonds in order of decreasing polarity:
C-H, N-H, O-H, and F-H.
(Multiple Choice)
4.9/5  (34)
(34)
Which is the best Lewis structure of CH2FCO2H (connectivity correct as given)?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following is not a valid resonance structure for N3-?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following are possible Lewis structures for a molecule with the formula C2H6O? 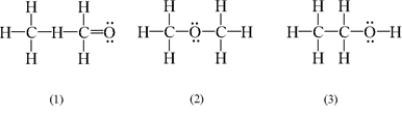
(Multiple Choice)
5.0/5  (35)
(35)
The nitrogen atom in the cyanide ion, CN-, is surrounded by:
(Multiple Choice)
4.8/5  (25)
(25)
When both of the electrons in a molecular bond originate from the same atom, the bond is called a(n)
(Multiple Choice)
4.9/5  (37)
(37)
What is the molecular geometry around the nitrogen atom as per the valence shell electron-pair repulsion (VSEPR) theory??
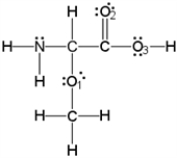
(Multiple Choice)
4.8/5  (34)
(34)
Showing 81 - 93 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)