Exam 7: The Structure of Atoms
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: the Tools of Quantitative Chemistry73 Questions
Exam 3: Atoms, Molecules, and Ions104 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions69 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure93 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals66 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids64 Questions
Exam 13: The Solid State67 Questions
Exam 14: Solutions and Their Behavior80 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions74 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria75 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases97 Questions
Exam 18: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria87 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy70 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions83 Questions
Exam 21: Environmental Chemistry: Earths Environment, Energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements81 Questions
Exam 23: The Chemistry of the Transition Elements80 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry40 Questions
Exam 26: Nuclear Chemistry189 Questions
Select questions type
Which of the following is/are correct postulates of Bohr's theory of the hydrogen atom ?
1.The energy of an electron in an atom is quantized (i.e. only specific energy values are possible).
2.The principal quantum number (n), specifies each unique energy level.
3.An electron transition from a lower energy level to a higher energy level results in an emission of a photon of light.
(Multiple Choice)
4.9/5  (35)
(35)
What is the value of the spin quantum number for an electron in a 3d orbital?
(Multiple Choice)
4.9/5  (29)
(29)
The energy required to break one mole of fluorine-fluorine bonds in F2 is 155 kJ/mol. What is the longest wavelength of light capable of breaking a single F-F bond?
(Multiple Choice)
4.8/5  (43)
(43)
For which of the following transitions would a hydrogen atom emit the lowest energy photon?
(Multiple Choice)
4.7/5  (36)
(36)
What is the total number of subshells found in the n = 7 shell?
(Multiple Choice)
4.9/5  (39)
(39)
The ____ of a photon of light is ____ proportional to its frequency and ____ proportional to its wavelength.
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following orbital boundary surfaces represent d-orbitals? 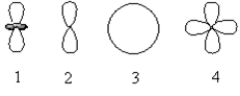
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following sets of quantum numbers (n, l, ml, ms) is not permissible?
(Multiple Choice)
4.8/5  (37)
(37)
What is the energy per mole of photons of light with a frequency of 9.01 × 1014 Hz?
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following ranks the regions of the electromagnetic spectrum from shortest to longest wavelength.
(Multiple Choice)
4.8/5  (52)
(52)
According to Heisenberg's _____ principle, it is impossible to simultaneously measure the exact location and energy of an electron.
(Short Answer)
4.8/5  (42)
(42)
What evidence does the photoelectric effect provide that photons are not only waves?
(Essay)
4.9/5  (38)
(38)
What is the value of the orbital angular momentum quantum number ( ) for an electron in a 4f orbital?
(Multiple Choice)
4.9/5  (32)
(32)
The Bohr model predicts that the energy of an atom's electron is _____. It means that the electron can only occupy orbitals of specific energies.
(Short Answer)
4.9/5  (31)
(31)
The electron in a hydrogen atom, originally in level n = 10, undergoes a transition to a lower level by emitting a photon of wavelength 1739 nm. What is the final level of the electron?
(c = 3.00 × 108 m/s, h = 6.626 × 10-34 J·s, RH = 2.179 × 10-18 J)
(Multiple Choice)
4.9/5  (41)
(41)
What is the energy of a photon of electromagnetic radiation with a frequency of Hz?
(Multiple Choice)
4.9/5  (34)
(34)
In Bohr's atomic theory, when an electron moves from one energy level to another energy level more distant from the nucleus,
(Multiple Choice)
4.8/5  (38)
(38)
What is the de Broglie wavelength of a 146-g baseball traveling at 99 mph? (1 mi = 1.609 km and h = 6.63 × 10-34 J·s)
(Multiple Choice)
4.9/5  (33)
(33)
Showing 21 - 40 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)