Exam 2: Lets Review: the Tools of Quantitative Chemistry
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: the Tools of Quantitative Chemistry73 Questions
Exam 3: Atoms, Molecules, and Ions104 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions69 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure93 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals66 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids64 Questions
Exam 13: The Solid State67 Questions
Exam 14: Solutions and Their Behavior80 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions74 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria75 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases97 Questions
Exam 18: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria87 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy70 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions83 Questions
Exam 21: Environmental Chemistry: Earths Environment, Energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements81 Questions
Exam 23: The Chemistry of the Transition Elements80 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry40 Questions
Exam 26: Nuclear Chemistry189 Questions
Select questions type
The volume of space occupied by a beryllium atom is 6.0 × 10-30 m3. What is this volume in units of nm3?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following volumes are equivalent to 75 L?
1) 75 cm3
2)7.5 × 104 mL
3)0.0075 kL
(Multiple Choice)
4.8/5  (36)
(36)
The SI unit for the diffusion coefficient is m2/s, but older texts sometimes report diffusion coefficients in units of in2/min. What is the value of a diffusion coefficient of 21.3 in2/min expressed in SI units? (2.54 cm = 1 in exactly)
(Multiple Choice)
4.8/5  (42)
(42)
A car averages 26.5 miles per gallon of gasoline. How many liters of gasoline will be needed for a trip of 621 km? Some conversion factors that may be helpful are the following: 1 qt = 0.946 L 1 mile = 1.609 km 4 qt = 1 gal (exact) 1 ft = 12 in (exact)
(Multiple Choice)
4.9/5  (44)
(44)
The melting point of a solid is 39 °F. This corresponds to _____.
(Multiple Choice)
4.8/5  (44)
(44)
How many feet (ft) make up 0.397 km if 1 mi = 1.609 km and 5280 ft = 1 mi?
(Multiple Choice)
4.8/5  (37)
(37)
Convert 42.12 cm3 to cubic inches (in3) given that 1 inch = 2.54 cm (exact).
(Multiple Choice)
4.8/5  (46)
(46)
The absolute zero point on the Kelvin scale is equal to _____.
(Multiple Choice)
4.7/5  (35)
(35)
The figure below depicts a shooting target. The black dots represent the shots fired by a shooter.
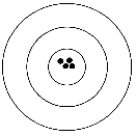 Which of the following best describes the shooter's results?
Which of the following best describes the shooter's results?
(Multiple Choice)
4.8/5  (37)
(37)
Assuming a large number of measurements is used to calculate the average of measurements, slightly more than _____ of the values collected are expected to be within two standard deviations
(Multiple Choice)
4.7/5  (39)
(39)
The number of significant figures in 2.1948 × 10-9 nm is _____.
(Multiple Choice)
4.8/5  (35)
(35)
A piece of metal (mass = 17.268 g) is placed in 10.50 mL of chloroform (d = 1.498 g/mL) in a 25-mL graduated cylinder. The chloroform level increases to 15.46 mL. The best value for density of this metal from these data is
(Multiple Choice)
4.8/5  (30)
(30)
Silver chloride (AgCl) is relatively insoluble in water. At 25 °C, 1.3 × 103 L of water is needed to dissolve 2.5 g of AgCl. What mass (in milligrams) of AgCl will dissolve in 1.0 L of water?
(Multiple Choice)
4.7/5  (39)
(39)
Which response has the correct number of significant figures and units for the following mathematical operation? ?
(Multiple Choice)
4.8/5  (32)
(32)
Convert 0.390 ng to milligrams and express the answer in scientific notation using the correct number of significant figures.
(Multiple Choice)
4.8/5  (42)
(42)
As the standard deviation of an average of several measurements increases, _____.
(Multiple Choice)
4.7/5  (39)
(39)
Carry out the following calculation and report your answer to the correct number of sig figs:
(Multiple Choice)
4.8/5  (27)
(27)
A 4.75 cm3 sample of solid gallium metal has a density of 5.910 g/cm3. What volume does this sample of gallium occupy in its liquid state? The density of liquid gallium is 6.100 g/cm3.
(Multiple Choice)
4.9/5  (41)
(41)
Showing 41 - 60 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)