Exam 1: Basic Concepts of Chemistry
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: the Tools of Quantitative Chemistry73 Questions
Exam 3: Atoms, Molecules, and Ions104 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions69 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure93 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals66 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids64 Questions
Exam 13: The Solid State67 Questions
Exam 14: Solutions and Their Behavior80 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions74 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria75 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases97 Questions
Exam 18: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria87 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy70 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions83 Questions
Exam 21: Environmental Chemistry: Earths Environment, Energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements81 Questions
Exam 23: The Chemistry of the Transition Elements80 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry40 Questions
Exam 26: Nuclear Chemistry189 Questions
Select questions type
Substances like hydrogen (H2) and oxygen (O2) that are composed of only one type of atom are classified as ________.
(Short Answer)
4.7/5  (26)
(26)
Which of the following is an example of qualitative information about a substance?
(Multiple Choice)
5.0/5  (37)
(37)
Which of following would be classified as a chemical change?
(Multiple Choice)
4.8/5  (35)
(35)
A pure substance composed of two or more different elements is a(n) ________.
(Multiple Choice)
4.9/5  (45)
(45)
A mass of 10 g of table salt dissolves in water to form a(n) ________ mixture (i.e., a mixture that is uniform throughout).
(Short Answer)
4.7/5  (40)
(40)
Which of the below figure represents a mixture of two elements?

(Multiple Choice)
4.8/5  (37)
(37)
Which of the following statements is true of a chemical equation?
(Multiple Choice)
4.9/5  (41)
(41)
To ensure integrity in science, experimental results should be ________ and reported in sufficient detail that the experiment can be repeated by others.
(Short Answer)
5.0/5  (33)
(33)
________ energy is the energy associated with the separation of two electrical charges.
(Short Answer)
4.9/5  (33)
(33)
A(n) ________ is a pure substance that is composed of only one type of atom.
(Multiple Choice)
4.9/5  (42)
(42)
Which one of the following is most likely to be a homogeneous mixture?
(Multiple Choice)
4.8/5  (46)
(46)
Which one of the following substances is classified as a chemical compound?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the below figures represents a homogeneous mixture?
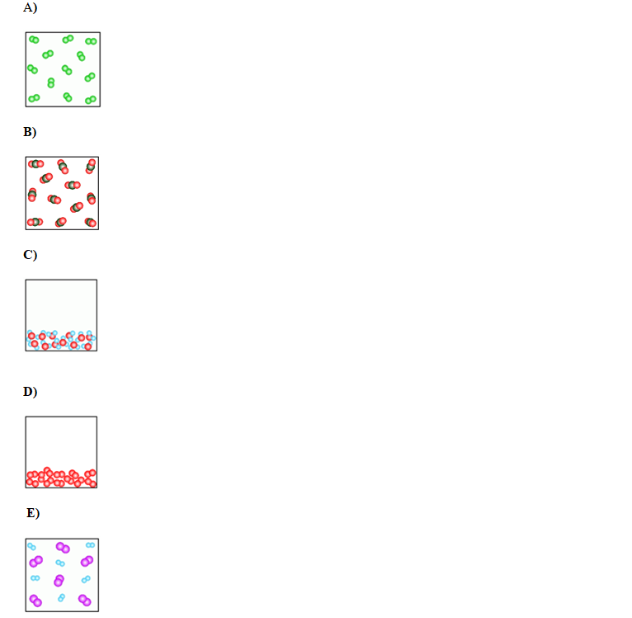
(Multiple Choice)
4.8/5  (41)
(41)
A(n) ________ is the smallest particle of an element that retains the characteristic chemical properties of that element.
(Short Answer)
4.9/5  (37)
(37)
Which of the following statements concerning green chemistry is not correct?
(Multiple Choice)
4.8/5  (33)
(33)
Properties, such as color and density, which can be observed or measured without changing the composition of a substance are called ________ properties.
(Short Answer)
4.8/5  (39)
(39)
Showing 21 - 40 of 40
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)