Exam 1: Chemistry - Methods and Measurement
Exam 1: Chemistry - Methods and Measurement104 Questions
Exam 2: The Structure of the Atom and the Periodic Table115 Questions
Exam 3: Structure and Properties of Ionic and Covalent Compounds93 Questions
Exam 4: Calculations, chemical Changes, and the Chemical Equation85 Questions
Exam 5: States of Matter: Gases, liquids, and Solids76 Questions
Exam 6: Solutions69 Questions
Exam 7: Energy,rate and Equilibrium67 Questions
Exam 8: Acids and Bases67 Questions
Exam 9: The Nucleus, radioactivity, and Nuclear Medicine86 Questions
Exam 10: An Introduction to Organic Chemistry104 Questions
Exam 11: The Unsaturated Hydrocarbons: Alkenes, alkynes, and Aromatics74 Questions
Exam 12: Alcohols, phenols, thiols, and Ethers69 Questions
Exam 13: Aldehydes and Ketones63 Questions
Exam 14: Carboxylic Acids and Carboxylic Acid Derivatives83 Questions
Exam 15: Amines and Amides82 Questions
Exam 16: Carbohydrates81 Questions
Exam 17: Lipids and Their Functions in Biochemical Systems78 Questions
Exam 18: Protein Structure and Function75 Questions
Exam 19: Enzymes74 Questions
Exam 20: Introduction to Molecular Genetics85 Questions
Exam 21: Carbohydrate Metabolism76 Questions
Exam 22: Aerobic Respiration and Energy Production75 Questions
Exam 23: Fatty Acid Metabolism75 Questions
Select questions type
What type of change alters the appearance,but not the composition or identity of the substance undergoing the change?
(Multiple Choice)
4.9/5  (40)
(40)
A student measures the mass of three separate samples of a solid: 104.45 g,0.838 g,and 46 g.If the student mixes all three samples together,how should the total mass be properly reported?
(Multiple Choice)
4.8/5  (33)
(33)
The area of a rectangle is determined by the formula: area = length × width.If a rectangle has a length of 32.6 cm and a width of 72.6 cm,what is the area of the rectangle to the correct number of significant figures?
(Multiple Choice)
4.8/5  (41)
(41)
Each piece of data is the individual result of a single measurement.
(True/False)
4.8/5  (37)
(37)
How many pounds are represented by 764.6 mg?
[1 pound = 454 g]
(Multiple Choice)
4.8/5  (32)
(32)
How many significant figures does the number 5.06305 × 104 contain?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following terms best describes the sample of matter in the diagram? Note: different colored circles represent atoms of different elements. 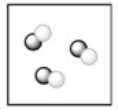
(Multiple Choice)
4.8/5  (42)
(42)
Air has an average density of 0.001226 g/mL.What volume of air would have a mass of 1.0 lb?
454 g = 1 pound
(Multiple Choice)
4.8/5  (27)
(27)
How should the result of the calculation below be reported using scientific notation and the proper number of significant figures?
(4.3169 × 104)÷ (2.02 × 103)= ?
(Multiple Choice)
4.9/5  (49)
(49)
Which of the following numbers has only one significant figure?
(Multiple Choice)
4.9/5  (47)
(47)
What is the number 0.0062985632 written in scientific notation to three significant figures?
(Multiple Choice)
4.8/5  (31)
(31)
What is the number 3,000 written in scientific notation using the proper number of significant figures?
(Multiple Choice)
4.7/5  (46)
(46)
Conversion of ice to liquid water or liquid water to steam is an example of what kind of change?
(Multiple Choice)
4.8/5  (35)
(35)
According to the Study Cycle,what is the recommended study session frequency?
(Multiple Choice)
4.9/5  (41)
(41)
Provide the answer to the following problem using scientific notation and the proper number of significant digits:
(6.00 × 10-2)(3.00 × 10-4)= ?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 21 - 40 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)