Exam 3: Structure and Properties of Ionic and Covalent Compounds
Exam 1: Chemistry - Methods and Measurement104 Questions
Exam 2: The Structure of the Atom and the Periodic Table115 Questions
Exam 3: Structure and Properties of Ionic and Covalent Compounds93 Questions
Exam 4: Calculations, chemical Changes, and the Chemical Equation85 Questions
Exam 5: States of Matter: Gases, liquids, and Solids76 Questions
Exam 6: Solutions69 Questions
Exam 7: Energy,rate and Equilibrium67 Questions
Exam 8: Acids and Bases67 Questions
Exam 9: The Nucleus, radioactivity, and Nuclear Medicine86 Questions
Exam 10: An Introduction to Organic Chemistry104 Questions
Exam 11: The Unsaturated Hydrocarbons: Alkenes, alkynes, and Aromatics74 Questions
Exam 12: Alcohols, phenols, thiols, and Ethers69 Questions
Exam 13: Aldehydes and Ketones63 Questions
Exam 14: Carboxylic Acids and Carboxylic Acid Derivatives83 Questions
Exam 15: Amines and Amides82 Questions
Exam 16: Carbohydrates81 Questions
Exam 17: Lipids and Their Functions in Biochemical Systems78 Questions
Exam 18: Protein Structure and Function75 Questions
Exam 19: Enzymes74 Questions
Exam 20: Introduction to Molecular Genetics85 Questions
Exam 21: Carbohydrate Metabolism76 Questions
Exam 22: Aerobic Respiration and Energy Production75 Questions
Exam 23: Fatty Acid Metabolism75 Questions
Select questions type
In the compound shown below,how can the bond between carbon and chlorine be described? 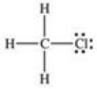
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
D
What is the name of CuF2 in the Stock system?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
E
What is the formula of iron(III)bromide?
Free
(Multiple Choice)
5.0/5  (43)
(43)
Correct Answer:
D
What term describes a solution of a compound in water that does not conduct an electric current?
(Multiple Choice)
4.7/5  (38)
(38)
What is the three-dimensional arrangement of positive and negative ions in an ionic solid called?
(Multiple Choice)
4.8/5  (35)
(35)
If the shape of a molecule is tetrahedral,what are the values of the bond angles?
(Multiple Choice)
4.9/5  (36)
(36)
As a rule,a polar substance will be a good solvent for nonpolar solutes,and vice versa.
(True/False)
4.8/5  (34)
(34)
Which of the following would NOT describe the Lewis structure of PF3?
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following compounds contains a polyatomic ion?
(Multiple Choice)
4.8/5  (38)
(38)
Which properly represents the Lewis structure of hydrogen sulfide,H2S?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 1 - 20 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)