Exam 9: Nucleophilic Substitution and Beta-Elimination
Exam 1: Covalent Bonding and Shapes of Molecules95 Questions
Exam 2: Alkanes and Cycloalkanes77 Questions
Exam 3: Stereoisomerism and Chirality75 Questions
Exam 4: Acids and Bases75 Questions
Exam 5: Alkenes: Bonding, Nomenclature, and Properties70 Questions
Exam 6: Reactions of Alkenes79 Questions
Exam 7: Alkynes80 Questions
Exam 8: Haloalkanes, Halogenation, and Radical Reactions58 Questions
Exam 9: Nucleophilic Substitution and Beta-Elimination89 Questions
Exam 10: Alcohols78 Questions
Exam 11: Ethers, Epoxides, and Sulfides71 Questions
Exam 12: Infrared Spectroscopy44 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy73 Questions
Exam 14: Mass Spectrometry39 Questions
Exam 15: An Introduction to Organometallic Compounds45 Questions
Exam 16: Aldehydes and Ketones94 Questions
Exam 17: Carboxylic Acids51 Questions
Exam 18: Functional Derivatives of Carboxylic Acids88 Questions
Exam 19: Enolate Anions and Enamines70 Questions
Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions44 Questions
Exam 21: Benzene and the Concept of Aromaticity59 Questions
Exam 22: Reactions of Benzene and Its Derivatives83 Questions
Select questions type
Which of the following statements related to SN1 reactions is not true?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following represents the transition state of the rate-determining step in the reaction between 2-chloropropane and sodium amide leading to elimination? 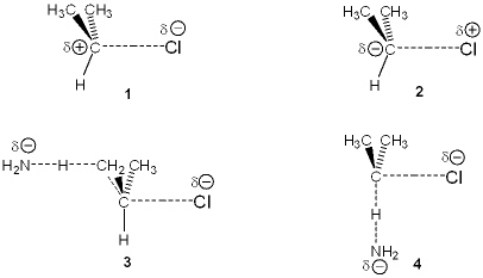
(Multiple Choice)
4.8/5  (42)
(42)
What is the equation for the rate of formation of 1-iodobutane from the reaction of 1-chlorobutane (BuCl) with NaI by an SN2 mechanism?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following alkyl bromides reacts the slowest with NaSCH3 in DMF? 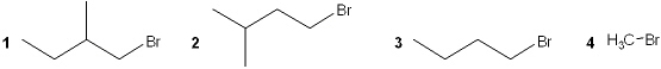
(Multiple Choice)
4.7/5  (30)
(30)
Which of the following sets consists of only polar aprotic solvents?
(Multiple Choice)
4.9/5  (39)
(39)
The reaction of tert-butyl bromide, (CH3)3CBr, with methanol in an inert solvent proceeds by an SN1 mechanism to give tert-butyl methyl ether, (CH3)3COCH3. What is the effect of doubling the concentration of methanol on the rate of the reaction?
(Multiple Choice)
4.7/5  (38)
(38)
What is the role of tetrabutylammonium chloride as a phase transfer catalyst in the reaction of 1-chlorooctane and sodium cyanide in a mixture of water and CH2Cl2?
(Multiple Choice)
4.9/5  (39)
(39)
What is the equation for the rate of formation of 2-methoxypropane, CH3CH(OCH3)CH3, from the reaction of 2-bromopropane (i-PrBr) with methanol?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following reactions corresponds to a substitution?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following alkyl halides undergoes the fastest solvolysis reaction with methanol, CH3OH?
(Multiple Choice)
4.8/5  (38)
(38)
Draw all of the chloroalkanes that undergo base-promoted dehydrochlorination to form the following alkene?
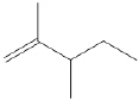
(Essay)
4.8/5  (41)
(41)
What is the major organic product obtained from the following reaction? 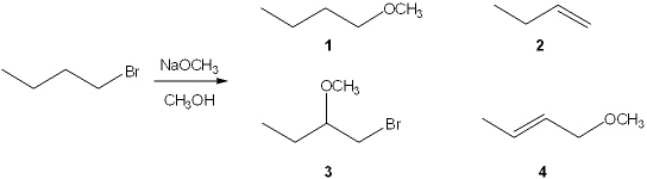
(Multiple Choice)
4.7/5  (40)
(40)
What is(are) the major organic product(s) obtained from the following substitution reaction? 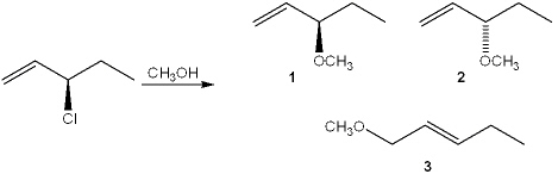
(Multiple Choice)
4.8/5  (46)
(46)
What is the major product formed upon treatment of (R) 2-bromohexane with sodium cyanide?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following represents the transition state of the rate-determining step in the reaction between tert-butyl bromide and methanol? 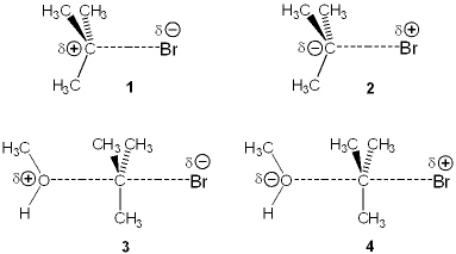
(Multiple Choice)
4.8/5  (39)
(39)
Draw all of the chloroalkanes that undergo base-promoted dehydrochlorination to form the following alkene?
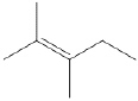
(Essay)
4.8/5  (40)
(40)
What is the major organic product obtained from the following reaction? 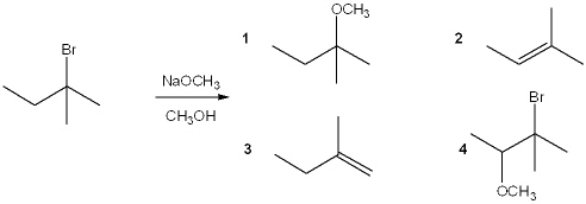
(Multiple Choice)
4.9/5  (45)
(45)
Which of the following statements related to SN1 reactions is not true?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following solvents is the best choice for the reaction of 1-chlorohexane with sodium bromide?
(Multiple Choice)
4.8/5  (46)
(46)
Which of the following statements is not true regarding SN2 reactions?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 61 - 80 of 89
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)