Exam 4: Acids and Bases
Exam 1: Covalent Bonding and Shapes of Molecules95 Questions
Exam 2: Alkanes and Cycloalkanes77 Questions
Exam 3: Stereoisomerism and Chirality75 Questions
Exam 4: Acids and Bases75 Questions
Exam 5: Alkenes: Bonding, Nomenclature, and Properties70 Questions
Exam 6: Reactions of Alkenes79 Questions
Exam 7: Alkynes80 Questions
Exam 8: Haloalkanes, Halogenation, and Radical Reactions58 Questions
Exam 9: Nucleophilic Substitution and Beta-Elimination89 Questions
Exam 10: Alcohols78 Questions
Exam 11: Ethers, Epoxides, and Sulfides71 Questions
Exam 12: Infrared Spectroscopy44 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy73 Questions
Exam 14: Mass Spectrometry39 Questions
Exam 15: An Introduction to Organometallic Compounds45 Questions
Exam 16: Aldehydes and Ketones94 Questions
Exam 17: Carboxylic Acids51 Questions
Exam 18: Functional Derivatives of Carboxylic Acids88 Questions
Exam 19: Enolate Anions and Enamines70 Questions
Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions44 Questions
Exam 21: Benzene and the Concept of Aromaticity59 Questions
Exam 22: Reactions of Benzene and Its Derivatives83 Questions
Select questions type
Which of the following energy diagrams best represents the changes in energy during addition of HBr to an alkene? 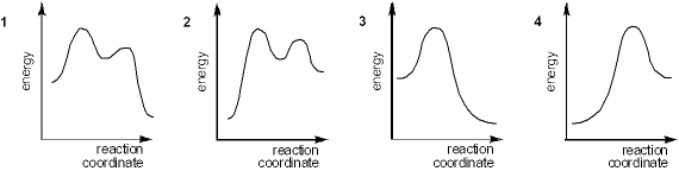
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
A
Which of the following terms describes the reactivity of trimethylamine, (CH3)3N?
Free
(Multiple Choice)
4.7/5  (42)
(42)
Correct Answer:
B
What is the approximate pKa value of acetic acid?
Free
(Multiple Choice)
4.9/5  (45)
(45)
Correct Answer:
B
Which set of curved arrows accounts for the deprotonation of an acid (A-H) by a base (:B)? 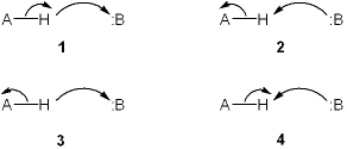
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following is a feature of a Brønsted-Lowry base?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following is present in the highest concentration upon dissolution of acetic acid in water?
(Multiple Choice)
4.7/5  (33)
(33)
Which of the following is a definition of the activation energy of a reaction?
(Multiple Choice)
4.9/5  (34)
(34)
Use curved arrows to show the movement of pairs of electrons in the following reaction between a Lewis acid and a Lewis base, and show the structure of the product.
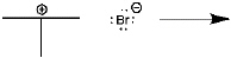
(Essay)
4.8/5  (42)
(42)
Which species is the conjugate acid in the following acid-base reaction? 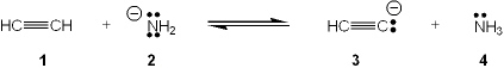
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following terms describes the reactivity of boron tribromide, BBr3?
(Multiple Choice)
4.7/5  (35)
(35)
Which of the following terms describes the role of ethanol in the acid-base reaction shown? 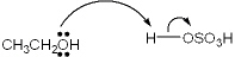
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following has a pKa value of approximately 16?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following energy diagrams represents the slowest reaction? 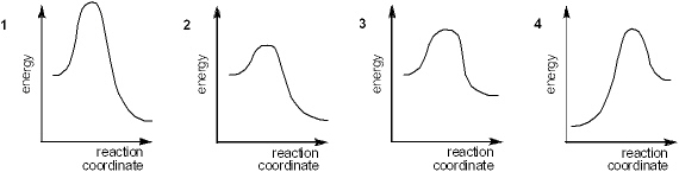
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following concepts can be used to rationalize the observation that acetic acid is a stronger acid than methanol?
(Multiple Choice)
4.8/5  (38)
(38)
What is the value of the equilibrium constant for the following equilibrium?
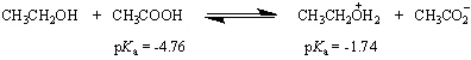
(Essay)
4.9/5  (41)
(41)
Under which of the following conditions will a reaction be spontaneous when DH > 0?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 75
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)