Exam 4: Acids and Bases
Exam 1: Covalent Bonding and Shapes of Molecules95 Questions
Exam 2: Alkanes and Cycloalkanes77 Questions
Exam 3: Stereoisomerism and Chirality75 Questions
Exam 4: Acids and Bases75 Questions
Exam 5: Alkenes: Bonding, Nomenclature, and Properties70 Questions
Exam 6: Reactions of Alkenes79 Questions
Exam 7: Alkynes80 Questions
Exam 8: Haloalkanes, Halogenation, and Radical Reactions58 Questions
Exam 9: Nucleophilic Substitution and Beta-Elimination89 Questions
Exam 10: Alcohols78 Questions
Exam 11: Ethers, Epoxides, and Sulfides71 Questions
Exam 12: Infrared Spectroscopy44 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy73 Questions
Exam 14: Mass Spectrometry39 Questions
Exam 15: An Introduction to Organometallic Compounds45 Questions
Exam 16: Aldehydes and Ketones94 Questions
Exam 17: Carboxylic Acids51 Questions
Exam 18: Functional Derivatives of Carboxylic Acids88 Questions
Exam 19: Enolate Anions and Enamines70 Questions
Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions44 Questions
Exam 21: Benzene and the Concept of Aromaticity59 Questions
Exam 22: Reactions of Benzene and Its Derivatives83 Questions
Select questions type
Which of the following energy diagrams represents the fastest reaction? 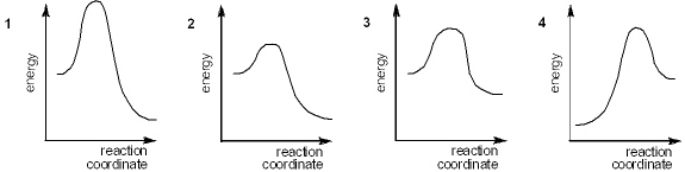
(Multiple Choice)
4.7/5  (30)
(30)
Which of the following is the correct order of decreasing basicity (stronger base > weaker base)?
(Multiple Choice)
4.9/5  (36)
(36)
Provide the equation that relates the acid dissociation constant, Ka, to the pKa of an acid.
(Short Answer)
4.8/5  (36)
(36)
Provide the equation for the equilibrium constant, Keq, for the following equilibrium.

(Essay)
4.9/5  (42)
(42)
Provide a brief explanation, based on features of the molecules, for the following trend in pKa values?

(Essay)
4.9/5  (34)
(34)
Which sets of curved arrows accounts for the protonation of propene with HI? 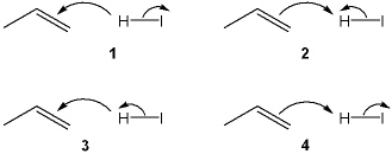
(Multiple Choice)
4.9/5  (31)
(31)
What is the value of the equilibrium constant for the following equilibrium?

(Essay)
5.0/5  (32)
(32)
What is the value of the equilibrium constant, Keq, for the following reaction? 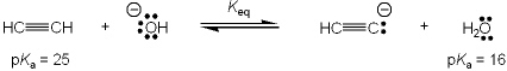
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following terms describes the role of ethene in the acid-base reaction shown? 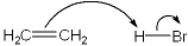
(Multiple Choice)
4.9/5  (44)
(44)
Provide a brief explanation, based on features of the molecules, for the following trend in pKa values?
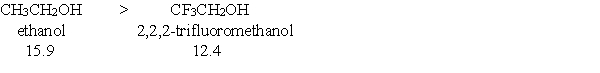
(Essay)
4.8/5  (40)
(40)
Use curved arrows to show the movement of pairs of electrons in the following acid-base reaction and show the structures of the conjugate acid and conjugate base.
(Essay)
4.8/5  (34)
(34)
Showing 61 - 75 of 75
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)