Exam 3: Introduction to Organic Molecules and Functional Groups
Exam 1: Structure and Bonding69 Questions
Exam 2: Acids and Bases52 Questions
Exam 3: Introduction to Organic Molecules and Functional Groups45 Questions
Exam 4: Alkanes57 Questions
Exam 5: Stereochemistry59 Questions
Exam 6: Understanding Organic Reactions45 Questions
Exam 7: Alkyl Halides and Nucleophilic Substitution61 Questions
Exam 8: Alkyl Halides and Elimination Reactions43 Questions
Exam 9: Alcohols, Ethers, and Related Compounds49 Questions
Exam 10: Alkenes43 Questions
Exam 11: Alkynes42 Questions
Exam 12: Oxidation and Reduction39 Questions
Exam 13: Mass Spectrometry and Infrared Spectroscopy35 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy29 Questions
Exam 15: Radical Reactions42 Questions
Exam 16: Conjugation, Resonance, and Dienes43 Questions
Exam 17: Benzene and Aromatic Compounds31 Questions
Exam 18: Reactions of Aromatic Compounds54 Questions
Exam 19: Carboxylic Acids and the Acidity of the O-H Bond36 Questions
Exam 20: Introduction to Carbonyl Chemistry;29 Questions
Exam 21: Aldehydes and Ketones Nucleophilic Addition42 Questions
Exam 22: Carboxylic Acids and Derivatives46 Questions
Exam 23: Substitution Reactions of Carbonyl Compounds at the Alpha-Carbon40 Questions
Exam 24: Carbonyl Condensation Reactions45 Questions
Exam 25: Amines53 Questions
Exam 26: Carbon-Carbon Bond Forming Reactions in Organic Synthesis37 Questions
Exam 27: Pericyclic Reactions47 Questions
Exam 28: Carbohydrates38 Questions
Exam 29: Amino Acids and Proteins35 Questions
Exam 30: Synthetic Polymers36 Questions
Exam 31: Lipids39 Questions
Select questions type
Why do heteroatoms confer reactivity on a particular molecule?
(Multiple Choice)
4.7/5  (44)
(44)
Which of the following molecules are aromatic hydrocarbons?
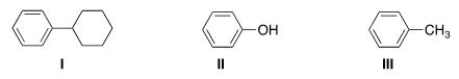
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following alkanes is expected to have the highest melting point?
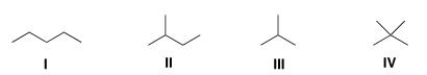
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following compounds has the highest boiling point?
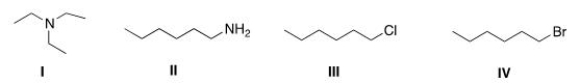
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following compounds is expected to be the least soluble in H2O?

(Multiple Choice)
4.9/5  (39)
(39)
Rank the following compounds in order of decreasing melting point, putting the compound with the highest melting point first. 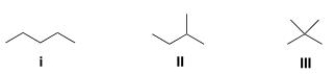
(Multiple Choice)
4.8/5  (50)
(50)
Rank the following compounds in order of increasing strength of intermolecular forces. 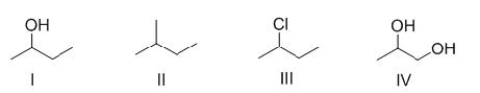
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following intermolecular forces would not form between similar molecules of the structure below?
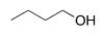
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following compounds has the highest boiling point?

(Multiple Choice)
4.8/5  (37)
(37)
Rank the following compounds in order of increasing strength of intermolecular forces, putting the molecule with the weakest intermolecular force first. 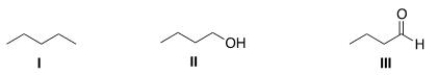
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following molecules contain the same functional groups?

(Multiple Choice)
4.7/5  (41)
(41)
What molecular features are required for soap to properly dissolve grease and oil?
(Multiple Choice)
4.7/5  (38)
(38)
Which of the following compounds is expected to be H2O soluble?
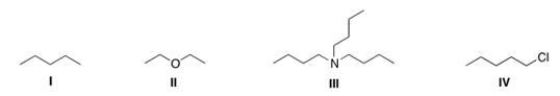
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following lists the correct functional groups found in aspartame, the artificial sweetener?
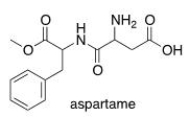
(Multiple Choice)
5.0/5  (36)
(36)
What intermolecular force is generally considered the strongest?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following structures contains a primary amine?
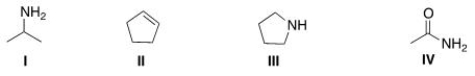
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following statements about the solubility of organic compounds in H2O is true?
(Multiple Choice)
4.7/5  (36)
(36)
Showing 21 - 40 of 45
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)