Exam 7: Electron Structure of the Atom
Exam 1: Matter and Energy110 Questions
Exam 2: Atoms, Ions, and the Periodic Table135 Questions
Exam 3: Chemical Compounds112 Questions
Exam 4: Chemical Composition111 Questions
Exam 5: Chemical Reactions and Equations108 Questions
Exam 6: Quantities in Chemical Reactions120 Questions
Exam 7: Electron Structure of the Atom116 Questions
Exam 8: Chemical Bonding114 Questions
Exam 9: The Gaseous State116 Questions
Exam 10: The Liquid and Solid States107 Questions
Exam 11: Solutions110 Questions
Exam 12: Reaction Rates and Chemical Equilibrium52 Questions
Exam 13: Acids and Bases128 Questions
Exam 14: Oxidation-Reduction Reactions117 Questions
Exam 15: Nuclear Chemistry88 Questions
Exam 16: Organic Chemistry119 Questions
Exam 17: Biochemistry95 Questions
Select questions type
Which of the following statements regarding orbital diagrams is correct?
(Multiple Choice)
4.8/5  (35)
(35)
The type of radiation with wavelengths just slightly longer than red light is infrared.
(True/False)
4.9/5  (36)
(36)
Rank the following elements in order of increasing atomic size: Ge, Rb, Ne, S
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following statements regarding the Bohr model of the hydrogen atom is incorrect?
(Multiple Choice)
4.8/5  (36)
(36)
Select the pair that has the larger atom or ion listed first.
(Multiple Choice)
4.8/5  (32)
(32)
Which element has the abbreviated ground-state electron configuration [Ar]4s23d6?
(Multiple Choice)
4.7/5  (32)
(32)
A laser used in DVD players has a wavelength of 405 nm.What is the frequency of this light in hertz (s-1)?
(Multiple Choice)
4.8/5  (32)
(32)
Rank the following elements in order of increasing atomic size: Sr, He, Si, F
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following is the correct ground state electron configuration for a magnesium atom?
(Multiple Choice)
4.8/5  (37)
(37)
The valence level and the number of valence electrons, respectively, for phosphorus are:
(Multiple Choice)
4.8/5  (43)
(43)
Sodium has a greater first ionization energy than cesium because sodium's valence electron is closer to the nucleus than cesium's valence electron.
(True/False)
4.9/5  (32)
(32)
The maximum number of electrons in the 2nd principal energy level is 8.
(True/False)
4.7/5  (32)
(32)
Which elements have a partially filled d sublevel in their ground-state electron configuration?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following is the correct orbital diagram for silicon?
(Multiple Choice)
4.9/5  (37)
(37)
The green line observed in the line spectrum for hydrogen has a wavelength of 486 nm.What is the energy of a photon of this light?
(Multiple Choice)
4.9/5  (35)
(35)
The following orbital diagram corresponds to the element___________. 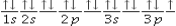
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following statements regarding valence electrons is incorrect?
(Multiple Choice)
4.9/5  (34)
(34)
Which element has the abbreviated ground-state electron configuration [Ar]4s23d8?
(Multiple Choice)
4.9/5  (41)
(41)
Showing 61 - 80 of 116
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)