Exam 22: The Atomic Nucleus
Exam 1: Physics and the Life Sciences40 Questions
Exam 2: Kinematics45 Questions
Exam 3: Forces40 Questions
Exam 4: Newtons Laws47 Questions
Exam 5: Centre of Mass and Linear Momentum42 Questions
Exam 6: Torque and Equilibrium48 Questions
Exam 7: Energy and Its Conservation48 Questions
Exam 8: Gases44 Questions
Exam 9: Thermal Physics52 Questions
Exam 10: Transport of Energy and Matter40 Questions
Exam 11: Static Fluids42 Questions
Exam 12: Fluid Flow47 Questions
Exam 13: Elasticity and Vibrations49 Questions
Exam 14: Waves40 Questions
Exam 15: Sound40 Questions
Exam 16: Electric Force and Field43 Questions
Exam 17: Electric Energy and Potential45 Questions
Exam 18: The Flow of Charges40 Questions
Exam 19: The Atom40 Questions
Exam 20: Magnetism and Electromagnetic Waves40 Questions
Exam 21: Geometric Optics41 Questions
Exam 22: The Atomic Nucleus47 Questions
Exam 23: Diagnostic Ultrasound Imaging40 Questions
Exam 24: Diagnostic X-Ray Imaging48 Questions
Exam 25: Diagnostic Nuclear Medicine Imaging34 Questions
Exam 26: Magnetic Resonance Imaging30 Questions
Exam 27: Radiation Therapy26 Questions
Select questions type
What kind of decay is the decay of 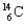 into
into 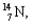 and which particles are being emitted?
and which particles are being emitted?
Free
(Essay)
4.9/5  (34)
(34)
Correct Answer:
â--decay, electron, and antineutrino
RAT: The atomic number increases, a neutron turns into a proton, and atomic mass remains the same.
Isotopic nuclides are nuclides with the same mass number.
Free
(True/False)
4.8/5  (40)
(40)
Correct Answer:
False
Which of these processes does NOT lead to a loss of charge in a nucleus?
Free
(Multiple Choice)
4.7/5  (38)
(38)
Correct Answer:
C
What is the fraction of nuclei from the initial radioactive sample that have decayed after 3 half-lives?
(Multiple Choice)
4.8/5  (34)
(34)
A radioactive sample is left to decay for 4 half-lives. The fraction of the sample that decayed is 1/16.
(True/False)
4.9/5  (41)
(41)
What kind of decay is the decay of 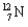 into
into 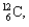 and which particles are being emitted?
and which particles are being emitted?
(Essay)
4.9/5  (42)
(42)
Describe briefly an isomer. What is the difference between 12Cg and 12Cm?
(Essay)
4.9/5  (42)
(42)
How does the mass of an atomic nucleus compare with the sum of masses of its constituent nucleons separated?
(Multiple Choice)
4.9/5  (34)
(34)
Discuss briefly how transition energy, ÄE, between two states of a spin- 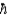 /2 nucleus in an external magnetic field of 1 T (f = 50 MHz) compares to the Balmer series in a H atom, for example, the electron transition between n2 and n3 levels that involves a photon of wavelength ë = 655 nm.
/2 nucleus in an external magnetic field of 1 T (f = 50 MHz) compares to the Balmer series in a H atom, for example, the electron transition between n2 and n3 levels that involves a photon of wavelength ë = 655 nm.
(Essay)
5.0/5  (37)
(37)
When an isomer decays, which of the following particles does it emit?
(Multiple Choice)
4.8/5  (31)
(31)
Using Table 22.1, calculate the energy released in the process of uranium decay: 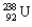 into
into 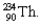
(Essay)
4.8/5  (39)
(39)
The Larmor frequency is the angular precession frequency of a nuclear spin in an external magnetic field.
(True/False)
4.9/5  (38)
(38)
Which of these processes does NOT lead to the loss of a nucleon from a nucleus?
(Multiple Choice)
4.8/5  (37)
(37)
Calculate the strength of an NMR magnet if the Larmor frequency of protons in that field is 100 MHz. The gyromagnetic ratio for protons is ã/2ð = 42.58 MHz/T.
(Short Answer)
4.8/5  (31)
(31)
Showing 1 - 20 of 47
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)