Exam 22: The Atomic Nucleus
Exam 1: Physics and the Life Sciences40 Questions
Exam 2: Kinematics45 Questions
Exam 3: Forces40 Questions
Exam 4: Newtons Laws47 Questions
Exam 5: Centre of Mass and Linear Momentum42 Questions
Exam 6: Torque and Equilibrium48 Questions
Exam 7: Energy and Its Conservation48 Questions
Exam 8: Gases44 Questions
Exam 9: Thermal Physics52 Questions
Exam 10: Transport of Energy and Matter40 Questions
Exam 11: Static Fluids42 Questions
Exam 12: Fluid Flow47 Questions
Exam 13: Elasticity and Vibrations49 Questions
Exam 14: Waves40 Questions
Exam 15: Sound40 Questions
Exam 16: Electric Force and Field43 Questions
Exam 17: Electric Energy and Potential45 Questions
Exam 18: The Flow of Charges40 Questions
Exam 19: The Atom40 Questions
Exam 20: Magnetism and Electromagnetic Waves40 Questions
Exam 21: Geometric Optics41 Questions
Exam 22: The Atomic Nucleus47 Questions
Exam 23: Diagnostic Ultrasound Imaging40 Questions
Exam 24: Diagnostic X-Ray Imaging48 Questions
Exam 25: Diagnostic Nuclear Medicine Imaging34 Questions
Exam 26: Magnetic Resonance Imaging30 Questions
Exam 27: Radiation Therapy26 Questions
Select questions type
The transition energy between two states of a spin- 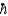 /2 nucleus in an external magnetic field is comparable to the Balmer series of electron transitions between energy states in the atom.
/2 nucleus in an external magnetic field is comparable to the Balmer series of electron transitions between energy states in the atom.
(True/False)
4.8/5  (35)
(35)
What is the maximal kinetic energy of an electron (â-particle) in the decay of sodium into magnesium?
[Note: ![What is the maximal kinetic energy of an electron (â-particle) in the decay of sodium into magnesium? [Note: + e<sup>-</sup> + v.]](https://storage.examlex.com/TB8418/11eb7f12_ec74_d2f8_bc0b_0d804afc83fd_TB8418_11.jpg)
![What is the maximal kinetic energy of an electron (â-particle) in the decay of sodium into magnesium? [Note: + e<sup>-</sup> + v.]](https://storage.examlex.com/TB8418/11eb7f12_ec74_fa09_bc0b_81b599b86a2c_TB8418_11.jpg)
![What is the maximal kinetic energy of an electron (â-particle) in the decay of sodium into magnesium? [Note: + e<sup>-</sup> + v.]](https://storage.examlex.com/TB8418/11eb7f12_ec74_fa0a_bc0b_7b810cef4d2b_TB8418_11.jpg) + e- + v.]
+ e- + v.]
(Essay)
4.8/5  (42)
(42)
Which of these changes occurs in the nucleus during beta decay?
(Multiple Choice)
4.7/5  (31)
(31)
The radioactive gas radon has a half-life of 3.8 days. How long will it take for the radon in a basement to decay to 50% of its present level?
(Multiple Choice)
4.8/5  (29)
(29)
A decay of 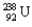 into
into 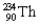 is an example of what type of a decay? What particle is released in the process?
is an example of what type of a decay? What particle is released in the process?
(Essay)
4.8/5  (27)
(27)
Nuclear force, like the electric force, acts only on charged particles. Neutrons are not affected by nuclear force.
(True/False)
4.8/5  (35)
(35)
The half-life is half the time needed for a radioactive sample to completely decay.
(True/False)
4.9/5  (31)
(31)
Which of these changes occurs in the nucleus during an alpha decay?
(Multiple Choice)
4.8/5  (30)
(30)
Which statement accurately describes the binding energy of a nucleus?
(Multiple Choice)
4.9/5  (33)
(33)
The activity of a radioactive sample is a positive or negative value of the decay rate, depending on the radioactive sample.
(True/False)
4.8/5  (37)
(37)
Initially there are 6000 nuclei of an isotope with a half-life of 3 hours. How many nuclei of that isotope will be left after 12 hours?
(Multiple Choice)
4.9/5  (32)
(32)
Calculate the energy released in the process of decay of ![Calculate the energy released in the process of decay of into [Note: 1 u = 931.5 MeV/c<sup>2</sup>.]](https://storage.examlex.com/TB8418/11eb7f12_ec74_abe4_bc0b_bbf42b9d4bda_TB8418_11.jpg) into
into ![Calculate the energy released in the process of decay of into [Note: 1 u = 931.5 MeV/c<sup>2</sup>.]](https://storage.examlex.com/TB8418/11eb7f12_ec74_abe5_bc0b_81e026acf88e_TB8418_11.jpg) [Note: 1 u = 931.5 MeV/c2.]
[Note: 1 u = 931.5 MeV/c2.]
(Essay)
4.9/5  (39)
(39)
Showing 21 - 40 of 47
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)