Exam 17: Quantisation and Wave-Particle Duality
Exam 1: Electric Fields46 Questions
Exam 2: Gausss Law44 Questions
Exam 3: Electric Potential49 Questions
Exam 4: Energy and Capacitance38 Questions
Exam 5: Current and Resistance33 Questions
Exam 6: Direct-Current Circuits49 Questions
Exam 7: Magnetic Fields45 Questions
Exam 8: Magnetic Forces50 Questions
Exam 9: Faradays Law49 Questions
Exam 10: Inductance23 Questions
Exam 11: Alternating-Current Circuits50 Questions
Exam 12: Electromagnetic Waves40 Questions
Exam 13: The Nature of Light and the Principles of Ray Optics38 Questions
Exam 14: Image Formation45 Questions
Exam 15: Wave Optics43 Questions
Exam 16: Diffraction Patterns and Polarisation44 Questions
Exam 17: Quantisation and Wave-Particle Duality41 Questions
Exam 18: Introduction to Quantum Mechanics43 Questions
Exam 19: Atomic Physics49 Questions
Exam 20: Quantum Physics of Molecules and Solids46 Questions
Exam 21: Nuclei and Radioactivity48 Questions
Exam 22: Particle Physics34 Questions
Select questions type
What is the shortest x-ray wavelength that can be produced in a 12-keV x-ray machine?
Free
(Short Answer)
4.9/5  (34)
(34)
Correct Answer:
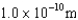
A photon collides with an electron.After the collision the wavelength of the scattered wave at a scattering angle greater than is: 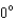
Free
(Multiple Choice)
4.7/5  (36)
(36)
Correct Answer:
D
The wavelength of a 45-kg teenager moving at 5 m/s on a skateboard is about:
Free
(Multiple Choice)
4.7/5  (47)
(47)
Correct Answer:
B
The threshold wavelength for photoelectric emission of a particular substance is 500 nm.What is the work function (in eV)?
(Multiple Choice)
4.8/5  (32)
(32)
Light is emitted by hydrogen atoms in the visible range for a hydrogen atom.Its wavelength is 656 nm.What value of n is associated with the light? (RH = 1.097 * 107 m-1)
(Multiple Choice)
4.9/5  (36)
(36)
An electron is accelerated through a potential difference of 25 000 V.What is the de Broglie wavelength of the electron (in m)?
(Multiple Choice)
4.8/5  (37)
(37)
What is the maximum kinetic energy (in eV) of a photoelectron emitted from a surface whose work function is 5.0 eV when illuminated by a light whose wavelength is 200 nm?
(Multiple Choice)
4.9/5  (31)
(31)
What is the maximum kinetic energy (in eV) of a photoelectron when a surface, whose work function is 5.0 eV, is illuminated by photons whose wavelength is 400 nm?
(Multiple Choice)
4.9/5  (29)
(29)
Your temperature is 98.6 F.Assuming your skin is a perfect radiator ( = 1), determine the wavelength corresponding to the largest intensity (in m).
(Multiple Choice)
4.9/5  (31)
(31)
Film behind a double slit is exposed to light in the following way: First one slit is opened and light is allowed to go through that slit for time t.Then it is closed and the other slit is opened and light is allowed to go through that slit for the same time t.When the film is developed the pattern will be:
(Multiple Choice)
4.7/5  (40)
(40)
What wavelength (in m) is associated with the Paschen series for n = 4? (RH = 1.097 * 107 m-1)
(Multiple Choice)
4.9/5  (35)
(35)
A solid state pulsed laser has an energy of 400 mJ per pulse.If its wavelength is 1.06 * 10-6 m, how many photons are in each pulse?
(Multiple Choice)
4.7/5  (25)
(25)
Photoelectrons are ejected when monochromatic light shines on a freshly-prepared (oxide-free) sodium surface.In order to obtain the maximum increase in the number of electrons ejected per second, the experimenter needs to:
(Multiple Choice)
4.9/5  (37)
(37)
What is the energy in eV of a photon of yellow light? = 500 nm.
(Short Answer)
4.9/5  (41)
(41)
A neutron has a mass of 1.67 *10-27 kg.The de Broglie wavelength is 1.4 * 10-10 m.How fast is the neutron going? (in m/s)
(Multiple Choice)
4.7/5  (36)
(36)
When a photon collides with a free electron at rest and the direction of motion of the photon changes:
(Multiple Choice)
4.8/5  (25)
(25)
An atomic level has a lifetime, 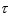 of.What is the linewidth, , of this level?
of.What is the linewidth, , of this level? 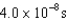
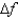
(Multiple Choice)
4.9/5  (32)
(32)
The experimental observation(s) below that require(s) a quantum explanation for the photoelectric effect:
(Multiple Choice)
4.8/5  (38)
(38)
If a rubidium surface is irradiated with blue light of wavelength 450.0 nm, what is the kinetic energy of the electrons emitted? (The work function for rubidium is = 2.09 eV.)
(Short Answer)
4.8/5  (37)
(37)
Showing 1 - 20 of 41
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)