Exam 20: Quantum Physics of Molecules and Solids
Exam 1: Electric Fields46 Questions
Exam 2: Gausss Law44 Questions
Exam 3: Electric Potential49 Questions
Exam 4: Energy and Capacitance38 Questions
Exam 5: Current and Resistance33 Questions
Exam 6: Direct-Current Circuits49 Questions
Exam 7: Magnetic Fields45 Questions
Exam 8: Magnetic Forces50 Questions
Exam 9: Faradays Law49 Questions
Exam 10: Inductance23 Questions
Exam 11: Alternating-Current Circuits50 Questions
Exam 12: Electromagnetic Waves40 Questions
Exam 13: The Nature of Light and the Principles of Ray Optics38 Questions
Exam 14: Image Formation45 Questions
Exam 15: Wave Optics43 Questions
Exam 16: Diffraction Patterns and Polarisation44 Questions
Exam 17: Quantisation and Wave-Particle Duality41 Questions
Exam 18: Introduction to Quantum Mechanics43 Questions
Exam 19: Atomic Physics49 Questions
Exam 20: Quantum Physics of Molecules and Solids46 Questions
Exam 21: Nuclei and Radioactivity48 Questions
Exam 22: Particle Physics34 Questions
Select questions type
The smallest object one can distinguish using the electron microscope is on the order of one nanometer (1 nm = 10-9 m).How many atoms of gold are contained in a cube whose edge is 1 nm long? The atomic mass of gold is 197 and its density is 19.3 g/cm3.
Free
(Short Answer)
4.8/5  (36)
(36)
Correct Answer:
59 atoms
When calculating the rotational kinetic energy of a diatomic molecule, with atoms of mass m1 and m2,the moment of inertia about an axis passing through the molecule's centre of mass, with r the atomic separation, is:
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
B
How many degrees of freedom does a diatomic molecule have?
Free
(Multiple Choice)
4.9/5  (43)
(43)
Correct Answer:
B
The Fermi temperature of copper is 80 000 K.The corresponding Fermi energy (in eV) is:
(Multiple Choice)
4.8/5  (39)
(39)
The force constant of HCl is 480 N/m.If the atomic masses are 1 u and 35 u (1 u = 1.66 *10-27 kg), find the fundamental frequency (in Hz).
(Multiple Choice)
4.8/5  (35)
(35)
A diatomic molecule consists of two point masses, m1 and m2, separated by a distance r.If x is the distance from m1 to the centre of mass, find the moment of inertia in terms of x about an axis parallel to the molecular axis through the centre of mass.
(Multiple Choice)
4.9/5  (44)
(44)
A diatomic molecule consists of two point masses, m1 and m2, separated by a distance r.Find the moment of inertia through the centre of mass about an axis perpendicular to the molecular axis.
(Multiple Choice)
4.9/5  (39)
(39)
The energy of a molecule can normally be divided into the following categories:
(Multiple Choice)
4.9/5  (40)
(40)
Solid argon has a density of 1650 kg/m3.The atomic weight of argon is 40.0.Assuming each atom occupies a cubical volume, what is the distance between the argon atoms?
(Short Answer)
5.0/5  (35)
(35)
Assume a diatomic molecule can be considered to be two point masses separated by a distance r.The centre of mass of the system is located a distance x from m1, equal to:
(Multiple Choice)
4.9/5  (40)
(40)
The frequency of a microwave absorbed by a molecule when changing from the J = 3 to J = 4 rotation energy state is 4.61 * 1011 Hz.The moment of inertia of the molecule (in kg . m2) is:
(Multiple Choice)
4.7/5  (39)
(39)
A diatomic molecule consists of two point masses, m1 and m2, separated by a distance r.If x is the distance from m1 to the centre of mass, find the moment of inertia in terms of x about an axis perpendicular to the molecular axis through the centre of mass.
(Multiple Choice)
5.0/5  (46)
(46)
What is the energy of the first rotational state of the hydrogen (H2) molecule? The separation between the protons is 10-10 m and the mass of each proton is 1.67 *10-27 kg.(h = 6.626 * 10-34 J . s and 1 eV = 1.6 *10-19 J.)
(Short Answer)
4.7/5  (42)
(42)
Assume the angular momentum of a diatomic molecule is quantised according to the relation .What are the allowed rotational kinetic energies? 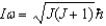
(Multiple Choice)
4.7/5  (35)
(35)
The fundamental frequency of HF is 8.72 * 1013 Hz.The energy associated with a transition from the 10th to the 9th vibrational quantum number (in eV) is:
(Multiple Choice)
4.9/5  (41)
(41)
The wave functions of some molecules are a combination of wave functions with different values of the orbital quantum number .The wave function of PF5 combines s, p and d states in an sp3d hybrid orbital.We would expect such an overlap of wave functions in individual molecules to represent: 
(Multiple Choice)
4.9/5  (45)
(45)
An oxygen molecule has a moment of inertia of 5 * 10-46 kg . m2.Calculate the bond length (in nm).Recall that the atomic mass of oxygen is 16 u (1 u = 1.66 * 10-27 kg).
(Multiple Choice)
4.9/5  (35)
(35)
Showing 1 - 20 of 46
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)