Exam 17: Acids, Bases, and Buffer Solutions: Life in an Aqueous Environment
Exam 18: Chemical Analysis: Characterizing Chemical Compounds26 Questions
Exam 17: Acids, Bases, and Buffer Solutions: Life in an Aqueous Environment21 Questions
Exam 16: Kinetics: What Affects the Speed of a Reaction20 Questions
Exam 15: Equilibria: How Far Do Reactions Go17 Questions
Exam 14: Energy: What Makes Reactions Go22 Questions
Exam 13: Reaction Mechanisms: The Chemical Changes That Drive the Chemistry of Life26 Questions
Exam 12: Chemical Reactions, Oxidation, and Reduction: Bringing Molecules to Life18 Questions
Exam 11: Metals in Biology: Life Beyond Carbon8 Questions
Exam 10: Biological Macromolecules: The Infrastructure of Life27 Questions
Exam 9: Isomerism: Generating Chemical Variety20 Questions
Exam 8: Molecular Shape and Structure: Life in Three Dimensions21 Questions
Exam 7: Functional Groups: Adding Function to the Framework of Life32 Questions
Exam 6: Hydrocarbons: The Framework of Life16 Questions
Exam 5: Moles, Concentrations, and Dilutions: Making Sense of Chemical Numbers22 Questions
Exam 4: Molecular Interactions: Holding It All Together15 Questions
Exam 3: Compounds and Chemical Bonding: Bringing Atoms Together21 Questions
Exam 2: Atoms: The Foundations of Life15 Questions
Exam 1: Introduction: Why Biologists Need Chemistry9 Questions
Select questions type
Match the following amino acids with the most appropriate description of the extent of ionization of their side chains when the pH of their environment has a value of 10.0
-Lysine (pKa 10.5)
Free
(Multiple Choice)
4.7/5  (37)
(37)
Correct Answer:
A
The pH of a 0.005 M solution of hydrobromic acid is which of the following?
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
C
A 0.1 M solution of ethanoic acid is 1.34% ionized. What is the value of Ka for ethanoic acid?
Free
(Multiple Choice)
4.8/5  (25)
(25)
Correct Answer:
B
Look at the following equilibrium reaction:
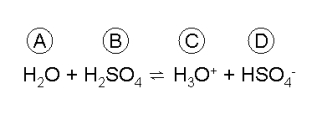 Match each chemical species with the term that best describes its role in this reaction scheme.
-Base
Match each chemical species with the term that best describes its role in this reaction scheme.
-Base
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following reactions depict the behaviour of an acid? Select any that apply.
(Multiple Choice)
4.8/5  (31)
(31)
Look at the following equilibrium reaction:
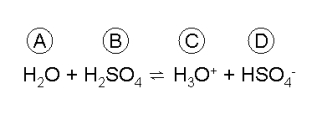 Match each chemical species with the term that best describes its role in this reaction scheme.
-Conjugate acid
Match each chemical species with the term that best describes its role in this reaction scheme.
-Conjugate acid
(Multiple Choice)
5.0/5  (41)
(41)
The pH of human blood is 7.4. Therefore the hydrogen ion concentration in human blood is which of the following?
(Multiple Choice)
4.9/5  (36)
(36)
Match the following amino acids with the most appropriate description of the extent of ionization of their side chains when the pH of their environment has a value of 10.0
-Glutamic acid (pKa 4.1)
(Multiple Choice)
5.0/5  (26)
(26)
Given the pKa for ammonium ion is 9.26, what is the pH of 1 L of
Solution which contains 5.35 g of NH4Cl, and contains the ammonium ion at a concentration of 0.2 mol L-1? (Molar mass of NH4Cl = 54.5 g mol-1.)
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following statements about buffer solutions are true? Select any that apply.
(Multiple Choice)
4.9/5  (33)
(33)
Look at the following equilibrium reaction:
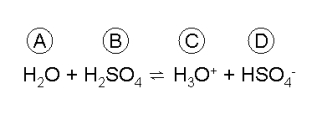 Match each chemical species with the term that best describes its role in this reaction scheme.
-Acid
Match each chemical species with the term that best describes its role in this reaction scheme.
-Acid
(Multiple Choice)
4.9/5  (34)
(34)
Match the following amino acids with the most appropriate description of the extent of ionization of their side chains when the pH of their environment has a value of 10.0
-Arginine (pKa 12.5)
(Multiple Choice)
4.9/5  (37)
(37)
If the concentration of [OH-] in an aqueous solution is 0.3 M, what is the pH of that solution?
(Multiple Choice)
4.9/5  (29)
(29)
Match the type of compound with the biological environment into which it is most likely to partition.
-Strong acid
(Multiple Choice)
4.8/5  (32)
(32)
A solution of HCl at concentration of 4 10-4 mol L-1 has a pH of which of the following?
(Multiple Choice)
4.9/5  (37)
(37)
Match the type of compound with the biological environment into which it is most likely to partition.
-Weak acid
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following acids would you use to make an effective buffer for a reaction at pH 8?
(Multiple Choice)
4.9/5  (38)
(38)
Which one of the following is the correct expression for the ion product of water?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 1 - 20 of 21
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)