Exam 16: Kinetics: What Affects the Speed of a Reaction
Exam 18: Chemical Analysis: Characterizing Chemical Compounds26 Questions
Exam 17: Acids, Bases, and Buffer Solutions: Life in an Aqueous Environment21 Questions
Exam 16: Kinetics: What Affects the Speed of a Reaction20 Questions
Exam 15: Equilibria: How Far Do Reactions Go17 Questions
Exam 14: Energy: What Makes Reactions Go22 Questions
Exam 13: Reaction Mechanisms: The Chemical Changes That Drive the Chemistry of Life26 Questions
Exam 12: Chemical Reactions, Oxidation, and Reduction: Bringing Molecules to Life18 Questions
Exam 11: Metals in Biology: Life Beyond Carbon8 Questions
Exam 10: Biological Macromolecules: The Infrastructure of Life27 Questions
Exam 9: Isomerism: Generating Chemical Variety20 Questions
Exam 8: Molecular Shape and Structure: Life in Three Dimensions21 Questions
Exam 7: Functional Groups: Adding Function to the Framework of Life32 Questions
Exam 6: Hydrocarbons: The Framework of Life16 Questions
Exam 5: Moles, Concentrations, and Dilutions: Making Sense of Chemical Numbers22 Questions
Exam 4: Molecular Interactions: Holding It All Together15 Questions
Exam 3: Compounds and Chemical Bonding: Bringing Atoms Together21 Questions
Exam 2: Atoms: The Foundations of Life15 Questions
Exam 1: Introduction: Why Biologists Need Chemistry9 Questions
Select questions type
Which of the following factors affect the rate of a chemical reaction? Select all that apply.
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
B,C,D
Match the type of enzyme inhibition with the observed effect on Vmax and KM
-Vmax decreases; KM decreases
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
C
Which of the following graphs depict a reaction proceeding at a constant rate? Select all that apply.
Free
(Multiple Choice)
4.8/5  (23)
(23)
Correct Answer:
A,B,C
Which of the following statements concerning the action of a catalyst are true? Select all that apply.
(Multiple Choice)
4.9/5  (38)
(38)
Which one of the following statements is false when an enzyme is saturated?
(Multiple Choice)
4.8/5  (33)
(33)
Match the type of enzyme inhibition with the observed effect on Vmax and KM
-Vmax decreases; KM is unchanged
(Multiple Choice)
4.8/5  (43)
(43)
When the temperature of a reaction is increased, the rate of the reaction is also increased. Which of the following is the correct reason for this?
(Multiple Choice)
4.8/5  (31)
(31)
The way in which the rate of a reaction depends on the concentration of its reactants is described by which of the following?
(Multiple Choice)
4.8/5  (36)
(36)
The concentration of a compound, X, is measured at different time intervals during the course of a chemical reaction. After plotting a graph of the concentration of X against time, shown in the graph below, the points labelled A-E on the graph have the following co-ordinates: A: x = 0 seconds; y = 0 mol L-1
B: x = 8 seconds; y = 0.37 mol L-1
C: x = 15 seconds; y = 0.49 mol L-1
D: x = 23 seconds; y = 0.63 mol L-1
E: x = 30 seconds; y = 0.62 mol L-1
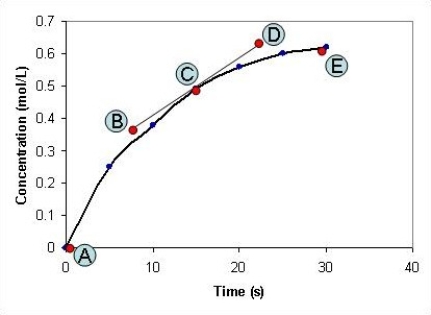 What is the rate of the chemical reaction after the reaction has been proceeding for fifteen seconds?
What is the rate of the chemical reaction after the reaction has been proceeding for fifteen seconds?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following correctly describes the effect of a catalyst on a chemical reaction? Select all that apply.
(Multiple Choice)
4.7/5  (33)
(33)
The activation energy of a reaction is equal to which of the following?
(Multiple Choice)
4.7/5  (36)
(36)
The following graph depicts the rate of an enzyme-catalyzed reaction. Which value, A-D, represents the Michaelis constant, KM, for the enzyme featured in the graph?
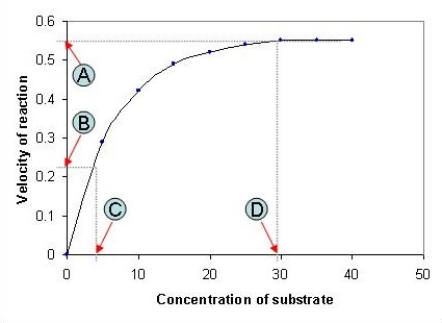
(Multiple Choice)
4.9/5  (32)
(32)
Look at the graph below. At which time point, A-D, is the reaction at its slowest?
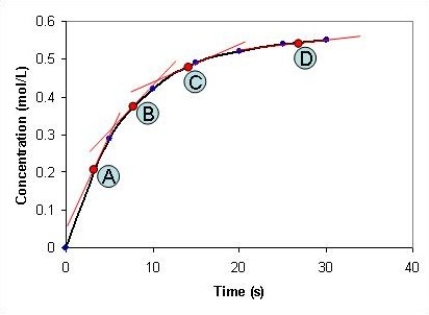
(Multiple Choice)
4.8/5  (36)
(36)
An enzyme-catalyzed reaction can be represented by which of the following general equations?
(Multiple Choice)
4.9/5  (39)
(39)
The units used to express the rate of a chemical reaction are which of the following?
(Multiple Choice)
4.8/5  (39)
(39)
The decomposition of a solution of hydrogen peroxide, H2O2, was monitored over several days. Initially, hydrogen peroxide was present at a concentration of 1 mol L-1; after four days the hydrogen peroxide was present at a concentration of 0.5 mol L-1. What is the rate of the reaction for the decomposition of hydrogen peroxide?
(Multiple Choice)
4.9/5  (29)
(29)
In order to function at maximum efficiency, enzymes often require which of the following? Select any that apply.
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following statements about the rate of a chemical reaction are true? Select all that apply.
(Multiple Choice)
4.9/5  (37)
(37)
Match the type of enzyme inhibition with the observed effect on Vmax and KM
-Vmax is unchanged; KM increases
(Multiple Choice)
4.8/5  (35)
(35)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)