Exam 5: Temperature and Heat
Exam 1: Measurement121 Questions
Exam 2: Motion97 Questions
Exam 3: Force and Motion105 Questions
Exam 4: Work and Energy106 Questions
Exam 5: Temperature and Heat105 Questions
Exam 6: Waves and Sound109 Questions
Exam 7: Optics and Wave Effects99 Questions
Exam 8: Electricity and Magnetism109 Questions
Exam 9: Atomic Physics173 Questions
Exam 10: Nuclear Physics174 Questions
Exam 11: The Chemical Elements179 Questions
Exam 12: Chemical Bonding156 Questions
Exam 13: Chemical Reactions150 Questions
Exam 14: Organic Chemistry166 Questions
Exam 15: Place and Time141 Questions
Exam 16: The Solar System104 Questions
Exam 17: Moons and Small Solar System Bodies149 Questions
Exam 18: The Universe191 Questions
Exam 19: The Atmosphere100 Questions
Exam 20: Atmospheric Effects97 Questions
Exam 21: Structural G Eology and P Late Tectonics161 Questions
Exam 22: Minerals, rocks, and Volcanoes170 Questions
Exam 23: Surface Processes121 Questions
Exam 24: Geologic Time148 Questions
Select questions type
An ideal gas is confined to a container with an adjustable volume.If the number of molecules and the temperature are held constant,by what factor will the volume change when the pressure is tripled?
(Short Answer)
4.8/5  (34)
(34)
What happens to a sample of water when it is heated between 0°C and 4°C?
(Multiple Choice)
4.9/5  (42)
(42)
How many kilocalories of heat would be needed to melt 0.12 kg of ice at 0°C and increase the temperature to 25°C?
(Short Answer)
4.9/5  (35)
(35)
A circular hole is drilled into an aluminum sheet.When the sheet is heated,the diameter of the hole will
(Multiple Choice)
4.9/5  (39)
(39)
On bare feet,a tile floor feels colder than a rug because the tile has a greater ______________.
(Short Answer)
5.0/5  (40)
(40)
The amount of heat necessary to change a liquid to a solid at constant temperature is the ______________.
(Short Answer)
4.9/5  (31)
(31)
A liter of water at room temperature (20°C)is heated to its boiling temperature.How many kilocalories of heat are required?
(Short Answer)
4.7/5  (34)
(34)
What happens to a sample of water when its temperature is reduced between 4°C and 100°C?
(Multiple Choice)
4.8/5  (42)
(42)
How much heat is necessary to change 10 g of ice at -20°C into water at 10°C?
(Multiple Choice)
4.8/5  (28)
(28)
A large calorie (Cal)is the amount of heat needed to change the temperature of one ______________ of water by one Celsius degree.
(Multiple Choice)
4.7/5  (37)
(37)
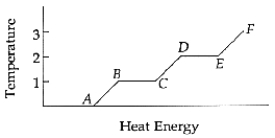 The latent heat of fusion is the amount of energy per kilogram necessary to go from point ______________ to point ______________.
The latent heat of fusion is the amount of energy per kilogram necessary to go from point ______________ to point ______________.
(Short Answer)
4.8/5  (34)
(34)
The number of molecules in a container is tripled and the Kelvin temperature doubled.The volume remains unchanged.The new pressure will be how many times greater than the original pressure?
(Short Answer)
4.8/5  (37)
(37)
When 10 kcal of heat is added to 2.0 kg of a substance,its temperature increases 20 C°.What is the specific heat of the substance?
(Short Answer)
4.8/5  (33)
(33)
The ______________ temperature scale has no negative readings.
(Short Answer)
4.8/5  (38)
(38)
How much heat is required to melt 5.0 kg of ice at 0°C to water at 0°C?
(Short Answer)
4.8/5  (41)
(41)
If 700 Joules of heat is added to a system,and the internal energy increases by 450 Joules,how much work is done by the system?
(Multiple Choice)
4.9/5  (29)
(29)
Showing 61 - 80 of 105
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)