Exam 22: All About Atoms
Exam 1: Vectors and Mesurement22 Questions
Exam 2: Gravitation, Force, Motion, Equilibrium, Elasticity, Rotation, Center of Mass, Linear Momentum, Potential Eneray, Conservation of Energy, Kinetic Energy, Work, Rolling, Torque, and Angular Momentum16 Questions
Exam 3: Oscillations, Fluids, Waves, Temperature, Heat, and the First Law of Thermodynamics13 Questions
Exam 4: The Kinetic Theory of Gases32 Questions
Exam 5: Entropy and the Second Law of Thermodynamics10 Questions
Exam 6: Coulombs Law8 Questions
Exam 7: Electric Fields8 Questions
Exam 8: Electric Potential and Gauss Law13 Questions
Exam 9: Capacitance17 Questions
Exam 10: Current and Resistance16 Questions
Exam 11: Circuits10 Questions
Exam 12: Magnetic Fields11 Questions
Exam 13: Magnetic Fields Due to Currents12 Questions
Exam 14: Induction and Inductance14 Questions
Exam 15: Electromagnetic Oscillations and Alternating Current17 Questions
Exam 16: Maxwells Equations: Magnetism of Matter20 Questions
Exam 17: Electromagnetic Waves20 Questions
Exam 18: Images17 Questions
Exam 19: Diffract and Interference14 Questions
Exam 20: Relativity18 Questions
Exam 21: More About Matter Waves, Photons and Matter Waves18 Questions
Exam 22: All About Atoms23 Questions
Exam 23: Conduction of Electricity in Solids12 Questions
Exam 24: Nuclear Physics24 Questions
Exam 25: Energy From the Nucleus20 Questions
Exam 26: Quarks, Leptons, and the Big Bang25 Questions
Select questions type
The effective charge acting on a single valence electron outside a closed shell is about Ne, where N is:
Free
(Multiple Choice)
4.8/5  (46)
(46)
Correct Answer:
C
The states being filled from the beginning to end of the lanthanide series of atoms are:
Free
(Multiple Choice)
4.8/5  (44)
(44)
Correct Answer:
D
Suppose the energy required to ionize an argon atom is i, the energy to excite it is e, and its thermal energy at room temperature is t. In increasing order, these three energies are:
Free
(Multiple Choice)
4.9/5  (44)
(44)
Correct Answer:
C
In a helium-neon laser, the laser light arises from a transition from a _________ state to a _________ state:
(Multiple Choice)
4.8/5  (38)
(38)
For any atom other that hydrogen and helium all electrons in the same shell have:
(Multiple Choice)
4.8/5  (43)
(43)
The most energetic electron in any atom at the end of a period of the periodic table is in:
(Multiple Choice)
4.9/5  (36)
(36)
An electron is in a quantum state for which there are seven allowed values of the z component of the angular momentum.The magnitude of the angular momentum is:
(Multiple Choice)
4.9/5  (32)
(32)
The magnetic field 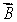 is along the z axis in a Stern-Gerlach experiment. The force it exerts on a magnetic dipole with dipole moment
is along the z axis in a Stern-Gerlach experiment. The force it exerts on a magnetic dipole with dipole moment 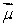 is proportional to:
is proportional to:
(Multiple Choice)
4.8/5  (33)
(33)
The group of atoms at the ends of periods of the periodic table are called:
(Multiple Choice)
4.8/5  (36)
(36)
The magnitude of the orbital angular momentum of an electron is what multiple of 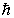 ? (
? ( 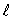 is a positive integer.)
is a positive integer.)
(Multiple Choice)
4.8/5  (40)
(40)
An electron in an atom is in a state with 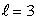 and
and 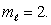 The angle between
The angle between 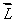 and the z axis is:
and the z axis is:
(Multiple Choice)
4.8/5  (40)
(40)
The group of atoms at the beginning of periods of the periodic table are called:
(Multiple Choice)
4.9/5  (36)
(36)
A magnetic dipole 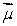 is placed in a strong uniform magnetic field
is placed in a strong uniform magnetic field 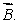 The associated force exerted on the dipole is:
The associated force exerted on the dipole is:
(Multiple Choice)
4.8/5  (42)
(42)
No state in an atom can be occupied by more than one electron. This is most closely related to the:
(Multiple Choice)
4.8/5  (31)
(31)
The most energetic electron in any atom at the beginning of a period of the periodic table is in:
(Multiple Choice)
4.8/5  (42)
(42)
Showing 1 - 20 of 23
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)