Exam 2: Acids and Bases Central to Understanding Organic Chemistry
Exam 1: Remembering General Chemistry Electronic Structure and Bonding4 Questions
Exam 2: Acids and Bases Central to Understanding Organic Chemistry10 Questions
Exam 3: An Introduction to Organic Compounds Nomenclature, Physical Properties, and Structure10 Questions
Exam 4: Isomers the Arrangement of Atoms in Space11 Questions
Exam 5: Alkenes Structure, Nomenclature, and an Introduction to Reactivity Thermodynamics and Kinetics8 Questions
Exam 6: The Reactions of Alkenes the Stereochemistry of Addition Reactions10 Questions
Exam 7: The Reactions of Alkynes an Introduction Tomultistep Synthesis12 Questions
Exam 8: Delocalized Electrons Their Eff11 Questions
Exam 9: Substitution and Elimination Reactions of Alkyl Halides11 Questions
Exam 10: Reactions of Alcohols, Ethers,epoxides, Amines, and Sulfur-Containing Compounds10 Questions
Exam 11: Organometallic Compounds10 Questions
Exam 12: Radicals10 Questions
Exam 13: Mass Spectrometry Infrared Spectroscopy Uv Vis Spectroscopy10 Questions
Exam 14: Nmr Spectroscopy12 Questions
Exam 15: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives12 Questions
Exam 16: Reactions of Aldehydes and Ketones More Reactions of Carboxylic Acid Derivatives11 Questions
Exam 17: Reactions at the Α-Carbon9 Questions
Exam 18: Reactions of Benzene and Substituted Benzenes11 Questions
Exam 19: More About Amines Reactions of Heterocyclic Compounds11 Questions
Exam 20: The Organic Chemistry of Carbohydrates11 Questions
Exam 21: Amino Acids, Peptides, and Proteins11 Questions
Exam 22: Catalysis in Organic Reactions and in Enzymatic Reactions10 Questions
Exam 23: The Organic Chemistry of the Coenzymes,compounds Derived From Vitamins11 Questions
Exam 24: The Organic Chemistry of the Metabolic Pathways11 Questions
Exam 25: The Organic Chemistry of Lipids7 Questions
Exam 26: The Chemistry of the Nucleic Acids9 Questions
Exam 27: Synthetic Polymers11 Questions
Exam 28: Pericyclic Reactions11 Questions
Select questions type
Which compound is the conjugate base of CH3OH?
Free
(Multiple Choice)
5.0/5  (27)
(27)
Correct Answer:
B
Which of the following is a Lewis acid?
Free
(Multiple Choice)
4.7/5  (39)
(39)
Correct Answer:
E
Which of the following is a factor in determining the acidity of a molecule?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
E
Consider the following equilibrium:
CH3C  CH + B ? CH3C
CH + B ? CH3C  C- + BH+
Which of the following bases would favor products in this reaction?
C- + BH+
Which of the following bases would favor products in this reaction?
(Multiple Choice)
4.8/5  (32)
(32)
What form of glutamic acid (below) predominates in a solution of
PH = 7.2?
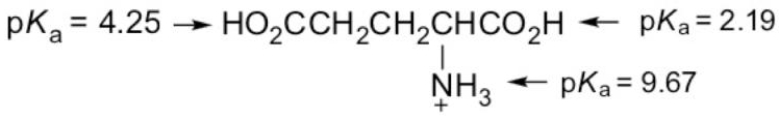
(Multiple Choice)
5.0/5  (41)
(41)
Which of the following acid-base reactions favor the products?
(Multiple Choice)
4.8/5  (32)
(32)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)