Exam 3: Life Has a Unique Chemistry
Exam 1: What Is Life41 Questions
Exam 2: Where Do We Come From and Where Do We Fit40 Questions
Exam 3: Life Has a Unique Chemistry49 Questions
Exam 4: Organization and Communication46 Questions
Exam 5: Tissues42 Questions
Exam 6: The Skeletomuscular System87 Questions
Exam 7: The Nervous System50 Questions
Exam 8: The Special Senses41 Questions
Exam 9: Immunity and the Lymphatic System58 Questions
Exam 10: Infectious Disease and Epidemiology41 Questions
Exam 11: Cancer46 Questions
Exam 12: The Cardiovascular System49 Questions
Exam 13: The Respiratory System: Movement of Air47 Questions
Exam 14: Nutrition: You Are What You Eat41 Questions
Exam 15: The Digestive System44 Questions
Exam 16: The Urinary System43 Questions
Exam 17: The Endocrine System and Development41 Questions
Exam 18: The Reproductive Systems: Maintaining the Species46 Questions
Exam 19: Pregnancy: Development From Conception to Newborn45 Questions
Exam 20: Inheritance, Genetics, and Molecular Biology41 Questions
Exam 21: Populations Evolve in Ecosystems52 Questions
Select questions type
Since carbon has 4 electrons in the outer energy level,it can form 4 covalent bonds.
(True/False)
4.9/5  (30)
(30)
A change in which ion concentration would severely affect heart rate and the nervous system?
(Multiple Choice)
4.8/5  (38)
(38)
Hydrogen bonds hold the hydrogen and oxygen together in water molecules.
(True/False)
4.8/5  (36)
(36)
The positively charged particle labeled A is called a(n)____. 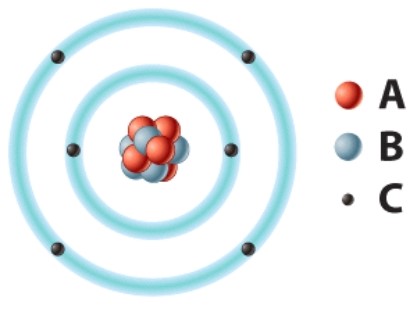
(Multiple Choice)
5.0/5  (31)
(31)
Which collection of elements are the most common in living organisms?
(Multiple Choice)
4.9/5  (44)
(44)
Which type of lipid is a key component of the cell membrane?
(Multiple Choice)
4.9/5  (31)
(31)
A positively charged ion is formed when an atom gains electrons.
(True/False)
4.9/5  (48)
(48)
Showing 21 - 40 of 49
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)