Exam 2: Basic Chemistry
Exam 1: The Human Body: An Orientation128 Questions
Exam 2: Basic Chemistry118 Questions
Exam 3: Cells and Tissues142 Questions
Exam 4: Skin and Body Membranes108 Questions
Exam 5: The Skeletal System124 Questions
Exam 6: The Muscular System129 Questions
Exam 7: The Nervous System161 Questions
Exam 8: Special Senses128 Questions
Exam 9: The Endocrine System149 Questions
Exam 10: Blood116 Questions
Exam 11: The Cardiovascular System295 Questions
Exam 13: The Respiratory System150 Questions
Exam 14: The Digestive System and Body Metabolism162 Questions
Exam 15: The Urinary System138 Questions
Exam 16: The Reproductive System169 Questions
Select questions type
Which of the following contains sodium?
Free
(Multiple Choice)
4.7/5  (38)
(38)
Correct Answer:
B
In order to break a disaccharide down into simple sugar units ________.
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
A
The four most common elements in the human body, in order of descending quantity, are hydrogen, carbon, oxygen, and nitrogen.
Free
(True/False)
4.9/5  (37)
(37)
Correct Answer:
False
What type of bond results when electrons are completely transferred from one atom to another?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following elements is needed to make functional thyroid hormone?
(Multiple Choice)
4.8/5  (27)
(27)
Polar molecules, like water, result when electrons are shared ________.
(Multiple Choice)
4.8/5  (39)
(39)
Glycogen and starch are examples of a specific category of carbohydrates called ________.
(Multiple Choice)
4.7/5  (38)
(38)
Unsaturated fatty acid chains contain one or more ________ bonds between carbon atoms.
(Multiple Choice)
4.8/5  (44)
(44)
The atomic number of an atom is equal to the number of ________ an atom contains.
(Multiple Choice)
4.8/5  (33)
(33)
The lower the pH, the greater the number of hydrogen ions released by a chemical into solution.
(True/False)
4.7/5  (52)
(52)
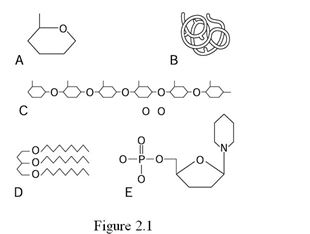 Using Figure 2.1, identify the following:
-Which letter represents a globular protein in its quaternary structure?
Using Figure 2.1, identify the following:
-Which letter represents a globular protein in its quaternary structure?
(Multiple Choice)
4.8/5  (40)
(40)
Match the following:
-Ionic bonds are formed when these subatomic particles are completely transferred from one atom to another atom.
(Multiple Choice)
4.9/5  (50)
(50)
Describe the difference between a polar and a nonpolar covalent bond. Give and explain an example of each type of bond.
(Essay)
4.8/5  (37)
(37)
Acids are defined as proton donors since they release hydrogen ions.
(True/False)
4.7/5  (32)
(32)
Showing 1 - 20 of 118
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)