Exam 2: Basic Chemistry
Exam 1: The Human Body: An Orientation128 Questions
Exam 2: Basic Chemistry118 Questions
Exam 3: Cells and Tissues142 Questions
Exam 4: Skin and Body Membranes108 Questions
Exam 5: The Skeletal System124 Questions
Exam 6: The Muscular System129 Questions
Exam 7: The Nervous System161 Questions
Exam 8: Special Senses128 Questions
Exam 9: The Endocrine System149 Questions
Exam 10: Blood116 Questions
Exam 11: The Cardiovascular System295 Questions
Exam 13: The Respiratory System150 Questions
Exam 14: The Digestive System and Body Metabolism162 Questions
Exam 15: The Urinary System138 Questions
Exam 16: The Reproductive System169 Questions
Select questions type
An atom with an atomic number of 14 will have ________ electrons in its valence shell.
(Multiple Choice)
4.9/5  (33)
(33)
A solution with a pH of 11.7 is ________ times more basic (alkaline) than a solution with a pH of 8.7.
(Multiple Choice)
5.0/5  (30)
(30)
A molecule of methane, CH₄, is known specifically as a(n) ________.
(Multiple Choice)
4.9/5  (45)
(45)
Elements are composed of building blocks known as ________.
(Multiple Choice)
4.8/5  (36)
(36)
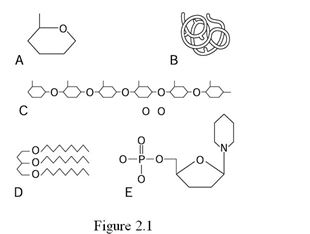 Using Figure 2.1, identify the following:
-Letter D represents the structure of a(n) ________.
Using Figure 2.1, identify the following:
-Letter D represents the structure of a(n) ________.
(Multiple Choice)
4.9/5  (39)
(39)
Match the following:
-Glycogen is broken down to release glucose subunits.
(Multiple Choice)
4.9/5  (43)
(43)
Atoms that have lost or gained electrons are known as ________.
(Multiple Choice)
4.9/5  (42)
(42)
The subatomic particles that are responsible for the chemical behavior of atoms are the ________.
(Multiple Choice)
4.9/5  (39)
(39)
The movement of ions across plasma membranes is an example of ________.
(Multiple Choice)
4.9/5  (38)
(38)
Hydrogen bonding between water molecules is responsible for ________.
(Multiple Choice)
4.8/5  (27)
(27)
Atoms that have lost or gained electrons during bonding are known as isotopes.
(True/False)
4.8/5  (37)
(37)
Match the following:
-Atoms that lose or gain these subatomic particles are known as ions.
(Multiple Choice)
4.7/5  (36)
(36)
Water absorbs and releases large amount of energy before changing temperature, a characteristic known as ________.
(Multiple Choice)
4.8/5  (39)
(39)
Match the following:
-Atoms share these subatomic particles equally in nonpolar covalent molecules.
(Multiple Choice)
4.8/5  (31)
(31)
The reaction sucrose + water → glucose + fructose is an example of a(n) ________.
(Multiple Choice)
5.0/5  (44)
(44)
Showing 21 - 40 of 118
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)