Exam 17: Acidbase Proton Transferreactions
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations48 Questions
Exam 4: Introduction to Gases56 Questions
Exam 5: Atomic Theory: The Nuclear Model of the Atom48 Questions
Exam 6: Chemical Nomenclature45 Questions
Exam 7: Chemical Formula Relationships45 Questions
Exam 8: Chemical Reactions44 Questions
Exam 9: Chemical Change52 Questions
Exam 10: Quantity Relationships in Chemical Reactions42 Questions
Exam 11: Atomic Theory: The Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications47 Questions
Exam 15: Gases, Liquids, and Solids45 Questions
Exam 16: Solutions47 Questions
Exam 17: Acidbase Proton Transferreactions45 Questions
Exam 18: Chemical Equilibrium45 Questions
Exam 19: Oxidationreduction Electron Transferreactions45 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
A solution is made by dissolving 0.0010 mole KOH in enough water to make 1.00 liter of solution.What are the pH and the pOH of the solution?
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
D
A pH meter is used to test the pH of a solution.See the figure below.  How is the solution classified?
How is the solution classified?
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
B
Given the following relative acid strengths,starting with the weakest: HCO3- < HNO3 < HBr,what is the relative strength of each conjugate base,starting with the weakest?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following statements is incorrect ?
i.Kw = [H+][OH-] = 1.0 × 10-14 at 25°C.
ii.Water or water solutions in which [H+] = [OH-] = 10-7 M are neutral solutions,neither acidic nor basic.
iii.A solution in which [H+] > [OH-] is basic
iv.A solution in which [OH- ] > [H+] is acidic
(Multiple Choice)
4.8/5  (43)
(43)
Given the following acid strengths: HF > H2S > HCN,which of the following reactions is predicted to occur from left to right as written?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following is not capable of acting like a Brønsted-Lowry base?
(Multiple Choice)
4.8/5  (45)
(45)
What is the hydroxide ion concentration in a solution with pH = 3?
(Multiple Choice)
4.9/5  (33)
(33)
Identify the conjugate acid base pairs in the reaction H2SO4 + HNO3 → H2NO3+ + HSO4-.
(Multiple Choice)
4.8/5  (38)
(38)
Consider the following image.  Based on the reading,what is the hydrogen ion concentration in this solution?
Based on the reading,what is the hydrogen ion concentration in this solution?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following is a Brønsted-Lowry base but not an Arrhenius base?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following properties is traditionally associated with Arrhenius bases?
(Multiple Choice)
4.8/5  (40)
(40)
Consider the following image which depicts the reactants in an acid-base reaction.Atoms other than H are labeled with the element symbol. 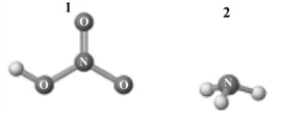 The products of this reaction are shown below.
The products of this reaction are shown below. 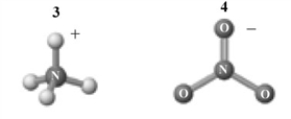 Which of the following is a correct interpretation of this reaction?
Which of the following is a correct interpretation of this reaction?
(Multiple Choice)
4.7/5  (41)
(41)
The hydroxide ion concentration of a solution is 0.00001 moles per liter.What is the hydrogen ion concentration,and is the solution acidic or basic?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 45
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)