Exam 8: Energy From Electron Transfer
Exam 1: The Air We Breathe76 Questions
Exam 2: Protecting the Ozone Layer75 Questions
Exam 3: The Chemistry of Global Climate Change73 Questions
Exam 4: Energy From Combustion69 Questions
Exam 5: Water for Life74 Questions
Exam 6: Neutralizing the Threat of Acid Rain68 Questions
Exam 7: The Fires of Nuclear Fission72 Questions
Exam 8: Energy From Electron Transfer70 Questions
Exam 9: The World of Polymers and Plastics84 Questions
Exam 10: Manipulating Molecules and Designing Drugs70 Questions
Exam 11: Nutrition: Food for Thought58 Questions
Exam 12: Genetic Engineering and the Molecules of Life59 Questions
Select questions type
A NiCd battery uses nickel and cadmium to produce a potential difference. Using these equations, answer the following questions.
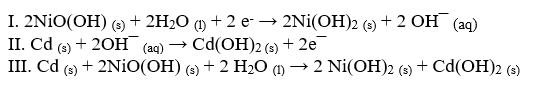 -Which equation represents the whole chemical reaction within the galvanic cell?
-Which equation represents the whole chemical reaction within the galvanic cell?
(Multiple Choice)
4.9/5  (38)
(38)
How do the interactions that are broken in water when it is boiled compare with those broken when water is electrolyzed?
(Multiple Choice)
4.8/5  (34)
(34)
Silicon has 4 electrons in its outer energy level,gallium has 3.Adding small amounts of gallium to pure silicon
(Multiple Choice)
4.9/5  (43)
(43)
Which is not a necessary consideration for a battery designed to run a cell phone or portable MP3 player?
(Multiple Choice)
4.8/5  (38)
(38)
Semiconductors are more effective than metals as converters of solar radiation into electricity because
(Multiple Choice)
4.8/5  (30)
(30)
Which argument(s)for the increasing use of solar energy is/are valid?
I.Solar cells are becoming cheaper and increasingly more efficient.
II.The cost of generating electricity from fossil fuels is increasing.
III.Limited and uncertain supply and the increasing requirements for pollution control are raising the cost of using fossil fuels.
(Multiple Choice)
4.9/5  (41)
(41)
What is a difference between solar energy from solar cells and solar energy from concentrating solar power (CSP)?
(Multiple Choice)
4.7/5  (41)
(41)
Showing 61 - 70 of 70
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)