Exam 8: Energy From Electron Transfer
Exam 1: The Air We Breathe76 Questions
Exam 2: Protecting the Ozone Layer75 Questions
Exam 3: The Chemistry of Global Climate Change73 Questions
Exam 4: Energy From Combustion69 Questions
Exam 5: Water for Life74 Questions
Exam 6: Neutralizing the Threat of Acid Rain68 Questions
Exam 7: The Fires of Nuclear Fission72 Questions
Exam 8: Energy From Electron Transfer70 Questions
Exam 9: The World of Polymers and Plastics84 Questions
Exam 10: Manipulating Molecules and Designing Drugs70 Questions
Exam 11: Nutrition: Food for Thought58 Questions
Exam 12: Genetic Engineering and the Molecules of Life59 Questions
Select questions type
Which is not a valid argument supporting the continued use of fossil fuels?
(Multiple Choice)
4.8/5  (35)
(35)
A NiCd battery uses nickel and cadmium to produce a potential difference. Using these equations, answer the following questions.
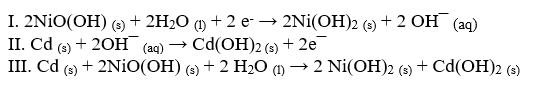 -Which equation represents what takes place at the anode?
-Which equation represents what takes place at the anode?
(Multiple Choice)
4.8/5  (43)
(43)
Which type of battery is best for use in heart (cardiac)pacemakers?
(Multiple Choice)
4.8/5  (37)
(37)
For safety and other practical reasons,the most logical use for hydrogen as a fuel in the near future is
(Multiple Choice)
4.9/5  (34)
(34)
Which of these locations would be best suited to geothermal energy as a source to operate homes?
(Multiple Choice)
4.8/5  (40)
(40)
The Prius,a hybrid car produced by Toyota,uses a battery that its maker claims should not have to be recharged or replaced during the lifetime of the car.The type of battery used in the Prius is
(Multiple Choice)
4.9/5  (46)
(46)
Batteries must be used in addition to solar cells when generating household electricity because
(Multiple Choice)
5.0/5  (40)
(40)
How does the reaction of hydrogen and oxygen to produce energy in a fuel cell differ from their interaction during the direct combustion of hydrogen and oxygen? 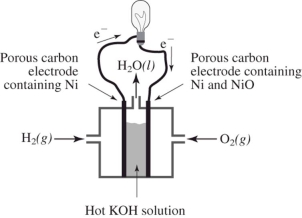 I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only water.
II.Much less heat energy is produced in a fuel cell than via direct combustion of hydrogen and oxygen.
III.In the fuel cell,there is an oxidation-reduction reaction between hydrogen and oxygen.In the direct combustion of hydrogen and oxygen,there is no such reaction.
IV.It is much easier to control the hydrogen and oxygen during direct combustion than during their reaction in a fuel cell.
I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only water.
II.Much less heat energy is produced in a fuel cell than via direct combustion of hydrogen and oxygen.
III.In the fuel cell,there is an oxidation-reduction reaction between hydrogen and oxygen.In the direct combustion of hydrogen and oxygen,there is no such reaction.
IV.It is much easier to control the hydrogen and oxygen during direct combustion than during their reaction in a fuel cell.
(Multiple Choice)
4.7/5  (40)
(40)
Semiconductors,such as the element silicon,may be used in cells that convert solar radiation to electricity.One of the major difficulties encountered in using silicon is that it
(Multiple Choice)
4.8/5  (35)
(35)
A candle may be considered a more efficient producer of light than a flashlight because it
(Multiple Choice)
4.8/5  (43)
(43)
Which increases the efficiency of a photovoltaic or solar cell?
I.Replacing crystalline silicon with its non-crystalline form.
II.Increasing the number of alternating p- and n-type layers of semiconductors.
III.Decreasing the thickness of each alternating p- and n-type layer of semiconductor.
(Multiple Choice)
4.8/5  (38)
(38)
At present,it will be difficult and perhaps inappropriate to develop an economy based on burning hydrogen rather than natural gas or gasoline because
(Multiple Choice)
4.8/5  (37)
(37)
Which has not been suggested as a reasonably practical way to store large amounts of hydrogen in relatively small spaces for its use as a fuel?
(Multiple Choice)
4.8/5  (42)
(42)
Which is not an advantage of using a solid electrolyte in a fuel cell?
(Multiple Choice)
4.8/5  (32)
(32)
A major advantage of a fuel cell over a standard battery is that
(Multiple Choice)
4.9/5  (34)
(34)
Showing 41 - 60 of 70
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)