Exam 12: Liquids, solids, and Intermolecular Forces
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases123 Questions
Exam 12: Liquids, solids, and Intermolecular Forces115 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry109 Questions
Exam 19: Biochemistry110 Questions
Select questions type
How many grams of C4H10O can be melted by 2.00 X 103 J?
Given 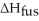 =7.27 kJ/mol
=7.27 kJ/mol
(Multiple Choice)
4.8/5  (34)
(34)
A situation where two opposite processes are occurring at equal rates,and no net change is taking place,is called:
(Multiple Choice)
4.8/5  (34)
(34)
For hydrogen bonding to occur,a molecule must have a hydrogen atom bonded directly to a fluorine,oxygen,or nitrogen atom.
(True/False)
4.9/5  (38)
(38)
How much energy does it take to melt a 16.87 g ice cube?
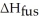 = 6.02 kJ/mol
= 6.02 kJ/mol
(Multiple Choice)
4.9/5  (37)
(37)
Rank the compounds NH3,CH4,and PH3 in order of increasing boiling point.
(Multiple Choice)
4.9/5  (40)
(40)
The measure of the resistance to the flow of a liquid is called:
(Multiple Choice)
4.9/5  (40)
(40)
Water has unusual physical properties which enable life to exist on earth.
(True/False)
4.8/5  (33)
(33)
A 250 gram sample of water at the boiling point had 45.0 kJ of heat added.How many grams of water were vaporized?
Heat of vaporization for water is 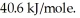
(Multiple Choice)
4.8/5  (39)
(39)
How many kJ of heat are needed to completely vaporize 3.30 moles of H2O?
The heat of vaporization for water at the boiling point is 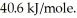
(Multiple Choice)
4.8/5  (36)
(36)
Which state of matter has a low density and is easily compressed?
(Multiple Choice)
4.8/5  (32)
(32)
Intermolecular forces determine if a substance is a solid,liquid or gas at room temperature.
(True/False)
4.7/5  (36)
(36)
Which statement is TRUE in describing what occurs when a solid melts to a liquid?
(Multiple Choice)
4.7/5  (36)
(36)
When one mole of water vapor at 100°C condenses,it will release an amount of energy equivalent to its heat of vaporization (40.7 kJ).
(True/False)
4.8/5  (47)
(47)
The rate of evaporation will increase if you pour a liquid out onto a large surface.
(True/False)
4.8/5  (37)
(37)
Increasing the intermolecular forces of a liquid will do which of the following?
(Multiple Choice)
4.9/5  (38)
(38)
How many kilojoules of heat are needed to completely vaporize 428 grams of C4H10O at its boiling point?
Given 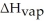 = 26.5kJ/mol
= 26.5kJ/mol
(Multiple Choice)
4.9/5  (43)
(43)
Showing 81 - 100 of 115
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)