Exam 9: Nucleophilic Substitution and Beta-Elimination
Exam 1: Covalent Bonding and Shapes of Molecules115 Questions
Exam 2: Alkanes and Cycloalkanes95 Questions
Exam 3: Stereoisomerism and Chirality93 Questions
Exam 4: Acids and Bases94 Questions
Exam 5: Alkenes: Bonding, Nomenclature, and Properties86 Questions
Exam 6: Reactions of Alkenes98 Questions
Exam 7: Alkynes100 Questions
Exam 8: Haloalkanes, Halogenation, and Radical Reactions76 Questions
Exam 9: Nucleophilic Substitution and Beta-Elimination111 Questions
Exam 10: Alcohols98 Questions
Exam 11: Ethers, Epoxides, and Sulfides93 Questions
Exam 12: Infrared Spectroscopy67 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy102 Questions
Exam 14: Mass Spectrometry65 Questions
Exam 15: An Introduction to Organometallic Compounds66 Questions
Exam 16: Aldehydes and Ketones119 Questions
Exam 17: Carboxylic Acids71 Questions
Exam 18: Functional Derivatives of Carboxylic Acids112 Questions
Exam 19: Enolate Anions and Enamines93 Questions
Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions69 Questions
Exam 21: Benzene and the Concept of Aromaticity85 Questions
Exam 22: Reactions of Benzene and Its Derivatives107 Questions
Exam 23: Amines88 Questions
Exam 24: Catalytic Carbon-Carbon Bond Formation77 Questions
Exam 25: Carbohydrates60 Questions
Exam 26: Lipids54 Questions
Exam 27: Amino Acids and Proteins70 Questions
Exam 28: Nucleic Acids52 Questions
Exam 29: Organic Polymer Chemistry60 Questions
Select questions type
Which of the following represents the transition state of the rate-determining step in the reaction between tert-butyl bromide and methanol? 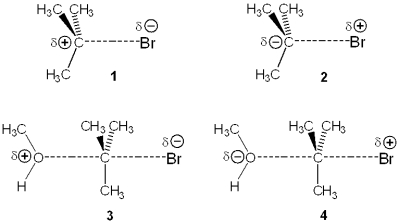
(Multiple Choice)
4.9/5  (34)
(34)
What is the equation for the rate of formation of tert-butyl alcohol from the reaction of tert-butyl bromide (t-BuBr) with water by an SN1 mechanism?
(Multiple Choice)
4.8/5  (33)
(33)
The reaction of tert-butyl bromide, (CH3)3CBr, with methanol in an inert solvent proceeds by an SN1 mechanism to give tert-butyl methyl ether, (CH3)3COCH3. What is the effect of doubling the concentration of methanol on the rate of the reaction?
(Multiple Choice)
4.8/5  (32)
(32)
What is the major organic product obtained from the following reaction? 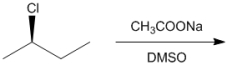
(Essay)
4.8/5  (37)
(37)
Which of the following is not a characteristic of SN2 reactions?
(Multiple Choice)
4.9/5  (41)
(41)
Consider the pair of reactions below to answer the following question(s).
-The solvent in these reactions would be classified as ______ ________.
(Short Answer)
4.7/5  (41)
(41)
Classify each of the following species ,Place the letter of the choice in the blank to the left of the formula.
-________ 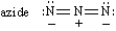
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following statements is not true regarding the SN2 reaction of (R)-2-bromobutane with sodium cyanide?
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following sets consists of only polar protic solvents?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following alkyl halides undergoes the fastest solvolysis reaction with ethanol, CH3CH2OH?
(Multiple Choice)
4.8/5  (38)
(38)
Draw all of the chloroalkanes that undergo base-promoted dehydrochlorination to form the following alkene? 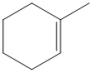
(Essay)
4.8/5  (34)
(34)
Draw all of the chloroalkanes that undergo base-promoted dehydrochlorination to form the following alkene? 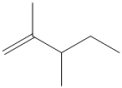
(Essay)
4.8/5  (43)
(43)
Provide a neatly drawn mechanism for the following reaction, including curved arrows to show the movement of pairs of electrons and the structure of reactive intermediates. 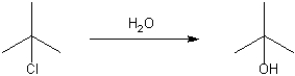
(Essay)
4.8/5  (42)
(42)
Which of the following statements related to SN1 reactions is not true?
(Multiple Choice)
4.7/5  (35)
(35)
What is the major product formed upon treatment of (R) 2-bromohexane with sodium cyanide?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following is not a characteristic of SN1 reactions?
(Multiple Choice)
4.8/5  (43)
(43)
What is the equation for the rate of formation of 2-methoxypropane, (CH3CH(OCH3)CH3, from the reaction of 2-bromopropane (i-PrBr) with sodium methoxide (NaOCH3)?
(Multiple Choice)
4.9/5  (40)
(40)
What is the major organic product obtained from the following reaction? 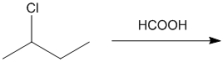
(Essay)
4.8/5  (35)
(35)
Which of the following energy diagrams represents the course of an exothermic E2 reaction? 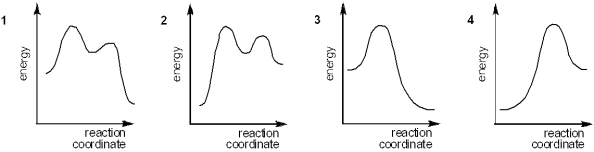
(Multiple Choice)
4.7/5  (41)
(41)
Which of the following alkyl halides undergoes the fastest solvolysis reaction with methanol, CH3OH?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 81 - 100 of 111
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)