Exam 3: An Introduction to Organic Compounds
Exam 1: Remembering General Chemistry: Electronic Structure and Bonding81 Questions
Exam 2: Acids and Bases: Central to Understanding Organic Chemistry37 Questions
Exam 3: An Introduction to Organic Compounds123 Questions
Exam 4: Isomers: the Arrangement of Atoms in Space111 Questions
Exam 5: Alkenes Thermodynamics and Kinetics76 Questions
Exam 6: Reactions of Alkenes93 Questions
Exam 7: Reactions of Alkynes Introduction to Multistep Synthesis119 Questions
Exam 8: Electron Delocalization, Resonance, and Aromaticity More About Molecular Orbital Theory166 Questions
Exam 9: Substitution Reactions of Alkyl Halides118 Questions
Exam 10: Elimination Reactions of Alkyl Halides Competition Between Substitution and Elimination94 Questions
Exam 11: Reactions of Alcohols, Ethers, Epoxides, and Sulfur-Containing Compounds100 Questions
Exam 12: Organometallic Compounds57 Questions
Exam 13: Reactions of Alkanes, Radicals130 Questions
Exam 14: Mass Spectrometry, Infrared Spectroscopy, and Uvvis Spectroscopy127 Questions
Exam 15: Nmr Spectroscopy110 Questions
Exam 16: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives128 Questions
Exam 17: Reactions of Aldehydes and Ketones116 Questions
Exam 18: Reactions at the Alpha Carbon of Carbonyl Compounds113 Questions
Exam 19: Reactions of Benzene and Substituted Benzenes155 Questions
Exam 20: More About Amines Heterocylic Compounds115 Questions
Exam 21: Carbohydrates100 Questions
Exam 22: Amino Acids, Peptides, and Proteins105 Questions
Exam 23: Catalysis85 Questions
Exam 24: The Organic Mechanisms of the Coenzymes92 Questions
Exam 25: The Chemistry of Metabolism94 Questions
Exam 26: Nucleosides, Nucleotides, and Nucleic Acids85 Questions
Exam 27: Synthetic Polymers106 Questions
Exam 28: Pericyclic Reactions92 Questions
Select questions type
Describe the source of angle strain and torsional strain present in cyclopropane.
Free
(Essay)
4.9/5  (39)
(39)
Correct Answer:
The angle strain arises from the compression of the ideal tetrahedral bond angle of 109.5° to 60°. The large torsional strain occurs since all C-H bonds on adjacent carbons are eclipsed.
Draw all possible constitutional isomers for C2H6O and give common names for each structure.
Free
(Essay)
4.7/5  (35)
(35)
Correct Answer:
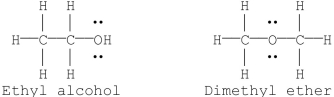
Identify the compound where the groups are axial and equatorial.
Free
(Multiple Choice)
4.9/5  (47)
(47)
Correct Answer:
B
Among the butane conformers, which occur at energy minima on a graph of potential energy versus dihedral angle?
(Multiple Choice)
4.9/5  (35)
(35)
Draw the most stable conformation of trans-1-tert-butyl-3-methylcyclohexane.
(Essay)
4.8/5  (32)
(32)
Classify the following amines as primary, secondary, or tertiary. 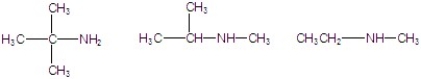
(Short Answer)
4.7/5  (40)
(40)
Arrange the following amines in order of increasing boiling point, lowest bp to highest bp: (CH3)2CHCH2CH2NH2, (CH3)2CHN(CH3)2, and (CH3)2CHCH2NHCH3.
(Essay)
4.9/5  (34)
(34)
What aspect of the fused ring systems present in cholesterol make it an ideal compound to lend rigidity to cell membranes?
(Essay)
4.9/5  (34)
(34)
Primary and secondary amines exhibit hydrogen bonding; tertiary amines do not. Explain.
(Essay)
4.8/5  (36)
(36)
Draw the chair conformer of cyclohexane. Label the axial hydrogens (Ha)and the equatorial hydrogens (He).
(Essay)
4.8/5  (39)
(39)
Draw the Newman structure for the most stable conformation of 1-bromopropane considering rotation about the C1-C2 bond.
(Essay)
4.8/5  (39)
(39)
View a butane molecule along the C2-C3 bond and provide a Newman projection of the lowest energy conformer.
(Essay)
4.8/5  (33)
(33)
Showing 1 - 20 of 123
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)