Exam 2: The Numerical Side of Chemistry
Exam 1: What Is Chemistry51 Questions
Exam 2: The Numerical Side of Chemistry147 Questions
Exam 3: The Evolution of Atomic Theory92 Questions
Exam 4: The Modern Model of the Atom97 Questions
Exam 5: Chemical Bonding and Nomenclature245 Questions
Exam 6: The Shape of Molecules92 Questions
Exam 7: Intermolecular Forces and the Phases of Matter67 Questions
Exam 8: Chemical Reactions75 Questions
Exam 9: Stoichiometry and the Mole191 Questions
Exam 10: Electron Transfer in Chemical Reactions87 Questions
Exam 11: What If There Were No Intermolecular Forces the Ideal Gas80 Questions
Exam 12: Solutions92 Questions
Exam 13: When Reactants Turn Into Products79 Questions
Exam 14: Chemical Equilibrium94 Questions
Exam 15: Electrolytes, Acids, and Bases94 Questions
Exam 16: Nuclear Chemistry84 Questions
Exam 17: The Chemistry of Carbon71 Questions
Exam 18: Synthetic and Biological Polymers47 Questions
Select questions type
A pound of feathers really does not weigh more than a pound of gold.
(True/False)
4.7/5  (36)
(36)
The commonly quoted distance from the earth to the sun (93,000,000 miles)is accurate to only 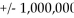 miles.
miles.
(True/False)
4.9/5  (36)
(36)
An aqueous salt solution may have a density that is less than 1g/mL.
(True/False)
4.9/5  (40)
(40)
When 20.5 grams is divided by 15 mL, the correct answer should be reported as ________.
(Multiple Choice)
4.9/5  (37)
(37)
The units of density for a liquid are usually expressed as ________.
(Multiple Choice)
4.9/5  (38)
(38)
The correct scientific notation for 147.8 × 10-3 is ________.
(Multiple Choice)
4.8/5  (40)
(40)
If the speed limit is 120 km/hr, its equivalent in mi/hr is ________.
(Multiple Choice)
4.9/5  (43)
(43)
Showing 61 - 80 of 147
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)