Exam 12: Acids and Bases
Exam 1: The Quantum World100 Questions
Exam 2: Quantum Mechanics in Action: Atoms100 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Molecular Shape and Structure95 Questions
Exam 5: The Properties of Gases94 Questions
Exam 6: Liquids and Solids95 Questions
Exam 7: Inorganic Materials100 Questions
Exam 8: Thermodynamics: The First Law94 Questions
Exam 9: Thermodynamics: The Second and Third Laws95 Questions
Exam 10: Physical Equilibria94 Questions
Exam 11: Chemical Equilibria94 Questions
Exam 12: Acids and Bases94 Questions
Exam 13: Aqueous Equilibria94 Questions
Exam 14: Electrochemistry94 Questions
Exam 15: Chemical Kinetics93 Questions
Exam 16: The Elements: the Main Group Elements189 Questions
Exam 17: The Elements: The D Block94 Questions
Exam 18: Nuclear Chemistry95 Questions
Exam 19: Organic Chemistry I94 Questions
Exam 20: Organic Chemistry II95 Questions
Select questions type
The fractional composition diagram for the amino acid alanine is given below. 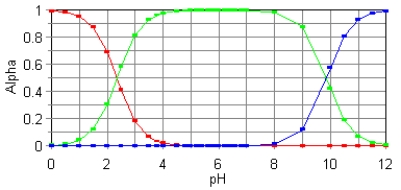 Write the structure of the dominant species at pH 1,6,and 12,respectively.
Write the structure of the dominant species at pH 1,6,and 12,respectively.
Free
(Essay)
4.9/5  (44)
(44)
Correct Answer:
HOOC-CH(CH3)NH3+,-OOC-CH(CH3)NH3+ and -OOC-CH(CH3)NH2.
Which of the following is the strongest base?
Free
(Multiple Choice)
4.8/5  (44)
(44)
Correct Answer:
A
Which of the following is the strongest acid?
Free
(Multiple Choice)
4.8/5  (50)
(50)
Correct Answer:
E
The pH of 0.010 M aniline(aq)is 8.32.What is the percentage aniline protonated?
(Multiple Choice)
4.7/5  (39)
(39)
In a solution labeled "0.0018 M barium hydroxide" what is the molarity of OH-?
(Multiple Choice)
4.9/5  (29)
(29)
The following 0.1 M aqueous solutions are arranged in order of increasing pH,with the highest pH on the far right.  Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
(Multiple Choice)
4.8/5  (37)
(37)
When CaO(s)is dissolved in water,which of the following is true?
(Multiple Choice)
4.8/5  (29)
(29)
The Ka of phenol is 1.3 * 10-10.For a solution labeled "1.0 * 10-3 M aqueous phenol,"
(Multiple Choice)
4.8/5  (33)
(33)
The amino acid alanine,HOOC-CH(CH3)NH3+,has Ka1 = 4.5 * 10-3 and Ka2 = 1.4 * 10-10.Calculate (-OOC-CH(CH3)NH3+)at pH 3.
(Multiple Choice)
4.8/5  (35)
(35)
All of the following acids have the same strength in water except
(Multiple Choice)
4.8/5  (39)
(39)
The amino acid methionine,HOOC-CH(CH2CH2SCH3)NH3+,has pKa1 = 2.2 and pKa2 = 9.1.If this amino acid is represented by H2L+,the major species at pH 6 is
(Multiple Choice)
4.7/5  (32)
(32)
Strong acids are leveled in water to the strength of the acid H3O+.
(True/False)
4.8/5  (35)
(35)
Write the charge balance equation for a solution that is 0.0010 M phenol(aq).Let phenol be represented by HA(aq).
(Multiple Choice)
4.9/5  (31)
(31)
The pH of 0.010 M H3PO4(aq)is 2.24; estimate the concentration of PO43- in the solution.For H3PO4,the values of Ka1,Ka2,and Ka3 are 7.6 *10-3,6.2 * 10-8,and 2.1 * 10-13,respectively.
(Multiple Choice)
4.9/5  (41)
(41)
Showing 1 - 20 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)