Exam 3: Acids and Bases
Exam 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties191 Questions
Exam 2: Molecular Representations168 Questions
Exam 3: Acids and Bases127 Questions
Exam 4: Alkanes and Cycloalkanes116 Questions
Exam 5: Stereoisomerism141 Questions
Exam 6: Chemical Reactivity and Mechanisms96 Questions
Exam 7: Alkyl Halides: Nucleophilic Substitution and Elimination Reactions224 Questions
Exam 8: Addition Reactions of Alkenes153 Questions
Exam 9: Alkynes177 Questions
Exam 10: Radical Reactions102 Questions
Exam 11: Synthesis106 Questions
Exam 12: Alcohols and Phenols147 Questions
Exam 13: Ethers and Epoxides; Thiols and Sulfides139 Questions
Exam 14: Infrared Spectroscopy and Mass Spectrometry135 Questions
Exam 15: Nuclear Magnetic Resonance Spectroscopy140 Questions
Exam 16: Conjugated Pi Systems and Pericyclic Reactions140 Questions
Exam 17: Aromatic Compounds118 Questions
Exam 18: Aromatic Substitution Reactions122 Questions
Exam 19: Aldehydes and Ketones169 Questions
Exam 20: Carboxylic Acids and Their Derivatives144 Questions
Exam 21: Alpha Carbon Chemistry: Enols and Enolates147 Questions
Exam 22: Amines112 Questions
Exam 23: Introduction to Organometallic Compounds118 Questions
Exam 24: Carbohydrates144 Questions
Exam 25: Amino Acids, Peptides, and Proteins133 Questions
Exam 26: Lipids123 Questions
Exam 27: Synthetic Polymers119 Questions
Select questions type
What is the conjugate base of phosphoric acid?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
B
Determine if NaOH is a suitable reagent to deprotonate the following compound. Explain why. 
Free
(Essay)
4.8/5  (34)
(34)
Correct Answer:
No.
The base has a negative charge on the more electronegative oxygen, whereas the conjugate base has a negative charge on the less electronegative carbon atom.
Identify the acid and the base and provide a curved arrow mechanism for the following reaction. 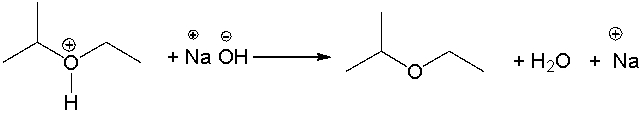
Free
(Essay)
4.8/5  (34)
(34)
Correct Answer:
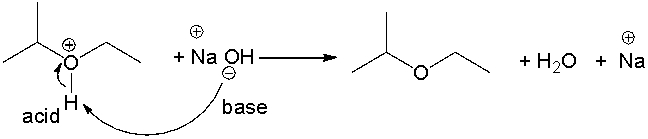
What is the strongest base that can be used with water as a solvent?
(Short Answer)
4.9/5  (32)
(32)
For the following reaction, which reactant functions as an acid? 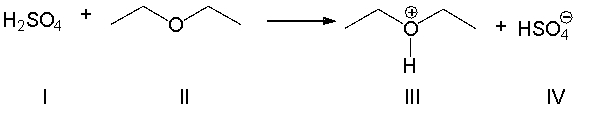
(Multiple Choice)
4.9/5  (37)
(37)
Identify the acid and the base and provide a curved arrow mechanism for the following reaction. 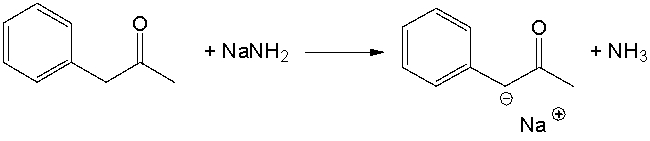
(Essay)
4.8/5  (40)
(40)
Provide a curved arrow mechanism for the following acid-base reaction. 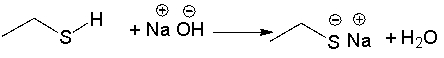
(Essay)
4.9/5  (44)
(44)
For the following acid-base reaction, predict which side of the equilibrium is favored. 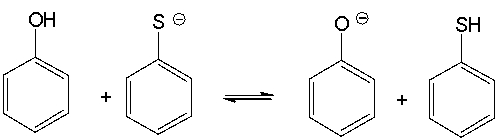
(Multiple Choice)
4.8/5  (40)
(40)
A gain of proton by a Brønsted-Lowry base results in a ___.
(Multiple Choice)
4.8/5  (39)
(39)
The following reaction mechanism contains mistakes. Which of the following statements correctly describes the curved arrows consistent with the reaction? 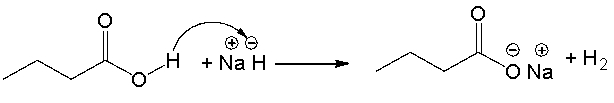
(Multiple Choice)
4.9/5  (33)
(33)
Determine if H2O is a suitable reagent to protonate the following compound. 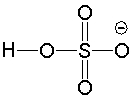
(Multiple Choice)
4.9/5  (38)
(38)
For the following reaction identify the acid and the base and predict the products. Draw the curved arrow mechanism for the formation of products and predict the direction of the equilibrium. 
(Essay)
4.7/5  (36)
(36)
Identify the Brønsted-Lowry base in the following reaction. 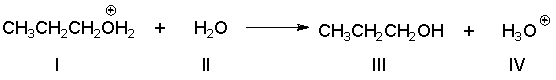
(Multiple Choice)
4.9/5  (38)
(38)
Showing 1 - 20 of 127
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)