Exam 3: Acids and Bases
Exam 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties191 Questions
Exam 2: Molecular Representations168 Questions
Exam 3: Acids and Bases127 Questions
Exam 4: Alkanes and Cycloalkanes116 Questions
Exam 5: Stereoisomerism141 Questions
Exam 6: Chemical Reactivity and Mechanisms96 Questions
Exam 7: Alkyl Halides: Nucleophilic Substitution and Elimination Reactions224 Questions
Exam 8: Addition Reactions of Alkenes153 Questions
Exam 9: Alkynes177 Questions
Exam 10: Radical Reactions102 Questions
Exam 11: Synthesis106 Questions
Exam 12: Alcohols and Phenols147 Questions
Exam 13: Ethers and Epoxides; Thiols and Sulfides139 Questions
Exam 14: Infrared Spectroscopy and Mass Spectrometry135 Questions
Exam 15: Nuclear Magnetic Resonance Spectroscopy140 Questions
Exam 16: Conjugated Pi Systems and Pericyclic Reactions140 Questions
Exam 17: Aromatic Compounds118 Questions
Exam 18: Aromatic Substitution Reactions122 Questions
Exam 19: Aldehydes and Ketones169 Questions
Exam 20: Carboxylic Acids and Their Derivatives144 Questions
Exam 21: Alpha Carbon Chemistry: Enols and Enolates147 Questions
Exam 22: Amines112 Questions
Exam 23: Introduction to Organometallic Compounds118 Questions
Exam 24: Carbohydrates144 Questions
Exam 25: Amino Acids, Peptides, and Proteins133 Questions
Exam 26: Lipids123 Questions
Exam 27: Synthetic Polymers119 Questions
Select questions type
Rank the following compounds in decreasing order of acidity. 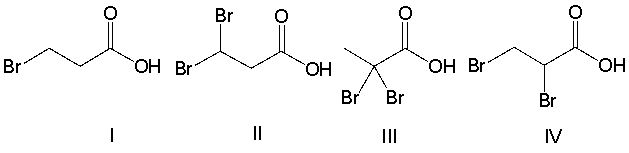
(Multiple Choice)
4.7/5  (43)
(43)
For the following reaction label the acid, base, conjugate acid and conjugate base. 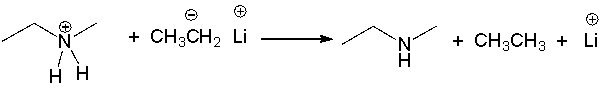
(Essay)
4.8/5  (33)
(33)
In a Brønsted-Lowry acid-base reaction, the reactants are _______.
(Multiple Choice)
4.8/5  (31)
(31)
Determine if NaNH2 is a suitable reagent to deprotonate the following compound. Explain why. 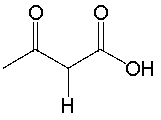
(Essay)
4.9/5  (42)
(42)
Provide a curved arrow mechanism for the following acid-base reaction. 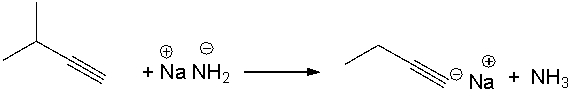
(Essay)
4.9/5  (37)
(37)
For the following acid-base reaction, predict which side of the equilibrium is favored. 
(Multiple Choice)
4.9/5  (30)
(30)
For the following acid-base reaction, predict which side the equilibrium is favored. Explain why. 
(Essay)
4.8/5  (41)
(41)
Showing 61 - 80 of 127
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)