Exam 17: Energy in Thermal Processes: the First Law of Thermodynamics
Exam 1: Introduction and Vectors69 Questions
Exam 2: Motion in One Dimension55 Questions
Exam 3: Motion in Two Dimensions82 Questions
Exam 4: The Laws of Motion97 Questions
Exam 5: More Applications of Newtons Laws80 Questions
Exam 6: Energy of a System81 Questions
Exam 7: Conservation of Energy77 Questions
Exam 8: Momentum and Collisions84 Questions
Exam 9: Relativity31 Questions
Exam 10: Rotational Motion121 Questions
Exam 11: Gravity,planetary Orbits,and the Hydrogen Atom62 Questions
Exam 12: Oscillatory Motion44 Questions
Exam 13: Mechanical Waves56 Questions
Exam 14: Superposition and Standing Waves57 Questions
Exam 15: Fluid Mechanics48 Questions
Exam 16: Temperature and the Kinetic Theory of Gases54 Questions
Exam 17: Energy in Thermal Processes: the First Law of Thermodynamics69 Questions
Exam 18: Heat Engines,entropy,and the Second Law of Thermodynamics51 Questions
Exam 19: Electric Forces and Electric Fields156 Questions
Exam 20: Electric Potential and Capacitance158 Questions
Exam 21: Current and Direct Current Circuits113 Questions
Exam 22: Magnetic Forces and Magnetic Fields157 Questions
Exam 23: Faradays Law and Inductance76 Questions
Exam 24: Electromagnetic Waves41 Questions
Exam 25: Reflection and Refraction of Light34 Questions
Exam 26: Image Formation by Mirrors and Lenses41 Questions
Exam 27: Wave Optics72 Questions
Exam 28: Quantum Physics69 Questions
Exam 29: Atomic Physics35 Questions
Exam 30: Nuclear Physics62 Questions
Exam 31: Particle Physics31 Questions
Select questions type
In which process will the internal energy of the system NOT change?
Free
(Multiple Choice)
4.7/5  (29)
(29)
Correct Answer:
B
How much heat (in kilocalories)is needed to convert 1.00 kg of ice at 0 C into steam at 100 C?
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
E
A 5-kg piece of lead (specific heat 0.03 cal/g. C)having a temperature of 80 C is added to 500 g of water having a temperature of 20 C.What is the final equilibrium temperature (in C)of the system?
(Multiple Choice)
4.9/5  (32)
(32)
When we say that the speed of sound is measured under adiabatic conditions we assume that
(Multiple Choice)
4.8/5  (31)
(31)
During an adiabatic compression,a volume of air decreases to 1/4 its original size.Calculate its final pressure if its original pressure was 1 atm.(Assume the air behaves like an ideal gas with = 1.4. )
(Multiple Choice)
4.8/5  (41)
(41)
A gas expands from A to B as shown in the graph.Calculate the work (in joules)done by the gas.(1 atm= 1.01 * 105 N/m2. ) 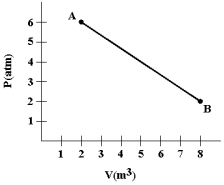
(Multiple Choice)
4.7/5  (37)
(37)
How much heat,in joules,is required to convert 1.00 kg of ice at 0 C into steam at 100 C? (Lice = 333 J/g;Lsteam = 2.26 *103 J/g. )
(Multiple Choice)
4.8/5  (26)
(26)
A 5-gallon container of water (approximately 20 kg)having a temperature of 212 F is added to a 50-gallon tub (approximately 200 kg)of water having a temperature of 50 F.What is the final equilibrium temperature (in C)of the mixture?
(Multiple Choice)
4.8/5  (35)
(35)
The specific heat of an ideal gas at constant pressure is greater than the specific heat of an ideal gas at constant volume because
(Multiple Choice)
5.0/5  (33)
(33)
How much water at 20 C is needed to melt 1 kilogram of solid mercury at its melting point of -39 C? (The heat of fusion of mercury is 2.8 cal/gram).
(Short Answer)
4.7/5  (40)
(40)
A 100 kg marble slab falls off a skyscraper and falls 200 m to the ground without hitting anyone.Its fall stops within milliseconds,so that there is no loss of thermal energy to its surroundings if its temperature is measured immediately after it stops.By how much has its temperature changed as a result of the fall,if we ignore energy gained or lost as a result of its interaction with the atmosphere? ( . )
(Multiple Choice)
4.9/5  (31)
(31)
An ideal gas is allowed to expand adiabatically until its volume increases by 50%.By approximately what factor is the pressure reduced? ( = 5/3. )
(Multiple Choice)
4.8/5  (40)
(40)
Water at room temperature,20 C,is pumped into a reactor core where it is converted to steam at 200 C.How much heat (in J)is transferred to each kilogram of water in this process? (csteam = 2 010 J/kg. C;Lsteam = 2.26 *103 J/g;1 cal = 4.186 J. )
(Multiple Choice)
4.8/5  (31)
(31)
How many calories of heat are required to raise the temperature of 4 kg of water from 50 F to the boiling point?
(Multiple Choice)
4.9/5  (28)
(28)
How much heat (in kcal)must be removed to make ice at -10 C from 2 kg of water at 20 C? (The specific heat of ice is 0.50 cal/g. C. )
(Multiple Choice)
4.9/5  (34)
(34)
A 5-g coin is dropped from a 300-m building.If it reaches a terminal velocity of 45 m/s,and the rest of the energy is converted to heating the coin,what is the change in temperature (in C)of the coin? (The specific heat of copper is 387 J/kg. C. )
(Multiple Choice)
4.8/5  (32)
(32)
A 100-kg student eats a 200-Calorie doughnut.To "burn it off",he decides to climb the steps of a tall building.How high (in m)would he have to climb to expend an equivalent amount of work? (1 food Calorie = 103 calories. )
(Multiple Choice)
4.8/5  (43)
(43)
An 8 000-kg aluminum flagpole 100-m long is heated by the sun from a temperature of 10 C to 20 C.Find the heat transferred (in J)to the aluminum if the specific heat of aluminum is 0.215 cal/g. C.
(Multiple Choice)
4.8/5  (33)
(33)
Showing 1 - 20 of 69
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)