Exam 19: The Second Law of Thermodynamics
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension83 Questions
Exam 3: Motion in Two and Three Dimensions60 Questions
Exam 4: Newtons Laws106 Questions
Exam 5: Applications of Newtons Laws73 Questions
Exam 6: Work and Energy60 Questions
Exam 7: Conservation of Energy56 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum92 Questions
Exam 9: Rotation105 Questions
Exam 10: Conservation of Angular Momentum66 Questions
Exam 11: Gravity84 Questions
Exam 12: Static Equilibrium and Elasticity58 Questions
Exam 13: Fluids77 Questions
Exam 14: Oscillations126 Questions
Exam 15: Wave Motion112 Questions
Exam 16: Superposition and Standing Waves87 Questions
Exam 17: Temperature and the Kinetic Theory of Gases78 Questions
Exam 18: Heat and the First Law of Thermodynamics100 Questions
Exam 19: The Second Law of Thermodynamics59 Questions
Exam 20: Thermal Properties and Processes50 Questions
Exam 21: The Electric Field I: Discrete Charge Distributions55 Questions
Exam 22: The Electric Field Ii: Continuous Charge Distributions64 Questions
Exam 23: Electric Potential87 Questions
Exam 24: Capacitance63 Questions
Exam 25: Electric Current and Direct-Current Circuits107 Questions
Exam 26: The Magnetic Field33 Questions
Exam 27: Sources of the Magnetic Field86 Questions
Exam 28: Magnetic Induction56 Questions
Exam 29: Alternating-Current Circuits106 Questions
Exam 30: Maxwells Equations and Electromagnetic Waves57 Questions
Exam 31: Properties of Light82 Questions
Exam 32: Optical Images106 Questions
Exam 33: Interference and Diffraction91 Questions
Exam 34: Wave Particle Duality and Quantum Physics140 Questions
Exam 35: Applications of the Schrodinger Equation42 Questions
Exam 36: Atoms113 Questions
Exam 37: Molecules39 Questions
Exam 38: Solids and the Theory of Conduction75 Questions
Exam 39: Relativity82 Questions
Exam 40: Nuclear Physics107 Questions
Exam 41: Elementary Particles and the Beginning of the Universe68 Questions
Select questions type
If a steam engine operates at half of its theoretical maximum efficiency (emax)and does work at a rate of W J/s,calculate how much heat is discharged per second.
Free
(Multiple Choice)
4.9/5  (28)
(28)
Correct Answer:
E
An engine operating in a cycle would violate the second law of thermodynamics if it
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
A
A heat engine absorbs 150 J of heat from a hot reservoir and rejects 90 J to a cold reservoir.What is the efficiency of this engine?
Free
(Multiple Choice)
4.7/5  (41)
(41)
Correct Answer:
B
A Carnot engine operating between reservoir temperatures of 340º C and 40ºC has an efficiency of
(Multiple Choice)
4.9/5  (36)
(36)
A refrigerator extracts heat Q from a cold reservoir.The heat exhausted to a hot reservoir
(Multiple Choice)
4.7/5  (35)
(35)
A Carnot heat engine absorbs heat Q from a hot reservoir at 127ºC and exhausts heat to a cold reservoir at 27ºC.How much heat is exhausted to the cold reservoir?
(Multiple Choice)
4.7/5  (37)
(37)
A heat engine absorbs 70 kcal of heat from a hot reservoir and exhausts 50 kcal to a cold reservoir each cycle.Its efficiency is
(Multiple Choice)
4.9/5  (41)
(41)
A refrigerator extracts 25 kJ from a cold reservoir and rejects 35 kJ to a hot reservoir.What is the coefficient of performance of this refrigerator?
(Multiple Choice)
5.0/5  (40)
(40)
You want to construct a perfect refrigerator from a perfect heat engine.What else do you need?
(Multiple Choice)
4.9/5  (32)
(32)
The change in the entropy of the universe due to an operating Carnot engine
(Multiple Choice)
4.9/5  (40)
(40)
A steam engine operates between a high and low temperature of 550 C and 180 C.If the steam engine operates at 40% of its theoretical maximum efficiency and does work at a rate of 1000 W,calculate how much heat is discharged per hour.
(Multiple Choice)
4.8/5  (29)
(29)
Two moles of a gas at T = 350 K expand quasistatically and isothermally from an initial volume of 20 L to a final volume of 60 L.The change in entropy of the gas during this expansion is (R = 8.314 J/mol·K)
(Multiple Choice)
4.7/5  (34)
(34)
Use the following diagram to answer the next problem. 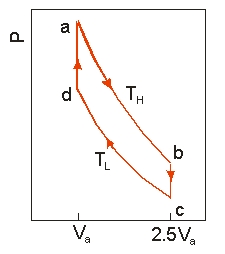 An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-How much heat is absorbed in going from a b?
An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-How much heat is absorbed in going from a b?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following statements is true of an isolated system consisting of 15 gas molecules?
(Multiple Choice)
4.8/5  (24)
(24)
Entropy is related to probability.An isolated system moves toward
(Multiple Choice)
4.7/5  (36)
(36)
Three moles of a gas at T = 250 K expand quasi-statically and adiabatically from an initial volume of 30 L to a final volume of 60 L.The change in entropy of the gas during this expansion is (R = 8.314 J/mol·K)
(Multiple Choice)
4.8/5  (29)
(29)
A heat engine exhausts heat Q to a cold reservoir.The amount of work done by the engine
(Multiple Choice)
4.9/5  (40)
(40)
The maximum theoretical thermal efficiency of a steam engine that is supplied steam at a temperature of 600ºC and exhausts it at a temperature of 200ºC is
(Multiple Choice)
4.8/5  (32)
(32)
Showing 1 - 20 of 59
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)