Exam 17: Temperature and the Kinetic Theory of Gases
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension83 Questions
Exam 3: Motion in Two and Three Dimensions60 Questions
Exam 4: Newtons Laws106 Questions
Exam 5: Applications of Newtons Laws73 Questions
Exam 6: Work and Energy60 Questions
Exam 7: Conservation of Energy56 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum92 Questions
Exam 9: Rotation105 Questions
Exam 10: Conservation of Angular Momentum66 Questions
Exam 11: Gravity84 Questions
Exam 12: Static Equilibrium and Elasticity58 Questions
Exam 13: Fluids77 Questions
Exam 14: Oscillations126 Questions
Exam 15: Wave Motion112 Questions
Exam 16: Superposition and Standing Waves87 Questions
Exam 17: Temperature and the Kinetic Theory of Gases78 Questions
Exam 18: Heat and the First Law of Thermodynamics100 Questions
Exam 19: The Second Law of Thermodynamics59 Questions
Exam 20: Thermal Properties and Processes50 Questions
Exam 21: The Electric Field I: Discrete Charge Distributions55 Questions
Exam 22: The Electric Field Ii: Continuous Charge Distributions64 Questions
Exam 23: Electric Potential87 Questions
Exam 24: Capacitance63 Questions
Exam 25: Electric Current and Direct-Current Circuits107 Questions
Exam 26: The Magnetic Field33 Questions
Exam 27: Sources of the Magnetic Field86 Questions
Exam 28: Magnetic Induction56 Questions
Exam 29: Alternating-Current Circuits106 Questions
Exam 30: Maxwells Equations and Electromagnetic Waves57 Questions
Exam 31: Properties of Light82 Questions
Exam 32: Optical Images106 Questions
Exam 33: Interference and Diffraction91 Questions
Exam 34: Wave Particle Duality and Quantum Physics140 Questions
Exam 35: Applications of the Schrodinger Equation42 Questions
Exam 36: Atoms113 Questions
Exam 37: Molecules39 Questions
Exam 38: Solids and the Theory of Conduction75 Questions
Exam 39: Relativity82 Questions
Exam 40: Nuclear Physics107 Questions
Exam 41: Elementary Particles and the Beginning of the Universe68 Questions
Select questions type
A hailstorm causes an average pressure of 1.4 N/m2 on the 200-m2 flat roof of a house.The hailstones,each of mass 7.0 10-3 kg,have an average velocity of 10 m/s perpendicular to the roof and rebound after hitting the roof with the same speed.How many hailstones hit the roof each second?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
B
The temperature of the air on a cold day when the temperature is 23ºF is
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
B
Five molecules of a gas have the following speeds:  The rms speed for these molecules is
The rms speed for these molecules is
Free
(Multiple Choice)
5.0/5  (26)
(26)
Correct Answer:
D
The air around us has 78% nitrogen and 21% oxygen.If the pressure is 1 atm,the pressure due to oxygen is
(Multiple Choice)
4.8/5  (37)
(37)
The relationship between the pressure and the volume of a gas expressed by Boyle's law holds true
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following is NOT an assumption made in the kinetic theory model of a gas?
(Multiple Choice)
4.9/5  (35)
(35)
A gas has a density X at standard temperature and pressure.What is the new density when the absolute temperature is doubled and the pressure increased by a factor of 3?
(Multiple Choice)
4.9/5  (29)
(29)
Two monoatomic gases,helium and neon,are mixed in the ratio of 2 to 1 and are in thermal equilibrium at temperature T (the molar mass of helium = 4.0 g/mol and the molar mass of neon = 20.2 g/mol).If the average kinetic energy of each helium atom is 6.3 10-21 J,calculate the temperature T.
(Multiple Choice)
4.8/5  (37)
(37)
A large balloon is being filled with He from gas cylinders.The temperature is 25 C and the pressure is 1 atmosphere.The volume of the inflated balloon is 2500 m3.What was the volume of He in the cylinders if the gas was under a pressure of 110 atmospheres and at a temperature of 12 C when in the gas cylinders?
(Multiple Choice)
4.9/5  (35)
(35)
If the rms speed of nitrogen molecules (molecular weight of N2 is 28 g/mol)at 273 K is 492 m/s,the rms speed of oxygen molecules (molecular weight of O2 is 32 g/mol)at the same temperature is approximately
(Multiple Choice)
5.0/5  (41)
(41)
At room temperature,which of the following diatomic molecules has the greater average kinetic energy: carbon monoxide (molar mass = 28 g/mol),nitrogen (molar mass = 28 g/mol),or oxygen (molar mass = 32 g/mol)?
(Multiple Choice)
4.8/5  (35)
(35)
Use the following to answer the question: 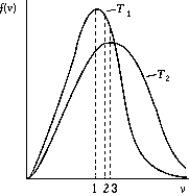 -The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
-The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
(Multiple Choice)
4.8/5  (35)
(35)
If the pressure and volume of an ideal gas are both reduced to half their original value,the absolute temperature of the gas is
(Multiple Choice)
4.9/5  (26)
(26)
A collection of oxygen molecules occupies a volume V at standard temperature and pressure.What is the new volume if the amount of oxygen is doubled and the pressure is tripled?
(Multiple Choice)
4.9/5  (34)
(34)
A mass of He gas occupies a volume V at standard temperature and pressure.What volume is occupied if the mass is halved,the absolute temperature doubled,and the pressure increased by a third?
(Multiple Choice)
4.8/5  (31)
(31)
If the rms speed of oxygen molecules is 460 m/s at 0ºC,the rms speed of oxygen molecules at 273ºC is approximately
(Multiple Choice)
4.8/5  (37)
(37)
The temperature at which the Celsius and Fahrenheit scales read the same is
(Multiple Choice)
4.9/5  (32)
(32)
Inside a sphere of radius 12 cm are 8.0 1023 gas molecules at a temperature of 50 C.What pressure do the gas molecules exert on the inside of the sphere?
(Multiple Choice)
4.9/5  (29)
(29)
Showing 1 - 20 of 78
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)