Exam 10: Acids and Bases and Equilibrium
Exam 1: Chemistry in Our Lives52 Questions
Exam 2: Chemistry and Measurement85 Questions
Exam 3: Matter and Energy110 Questions
Exam 4: Atoms and Elements106 Questions
Exam 5: Nuclear Chemistry90 Questions
Exam 6: Ionic and Molecular Compounds106 Questions
Exam 7: Chemical Quantities and Reactions114 Questions
Exam 8: Gases76 Questions
Exam 9: Solutions73 Questions
Exam 10: Acids and Bases and Equilibrium67 Questions
Exam 11: Introduction to Organic Chemistry: Hydrocarbons123 Questions
Exam 12: Alcohols, thiols, ethers, aldehydes, and Ketones78 Questions
Exam 13: Carbohydrates81 Questions
Exam 14: Carboxylic Acids, esters, amines, and Amides103 Questions
Exam 15: Lipids65 Questions
Exam 16: Amino Acids, proteins, and Enzymes90 Questions
Exam 17: Nucleic Acids and Protein Synthesis64 Questions
Exam 18: Metabolic Pathways and Energy Production131 Questions
Select questions type
According to the Arrhenius concept,if 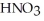 were dissolved in water,it would act as ________.
were dissolved in water,it would act as ________.
(Multiple Choice)
5.0/5  (43)
(43)
According to LeChâtelier's principle,predict whether adding H2O causes the system to shift in the direction of reactants,products,or no change. N 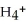 (aq)+
(aq)+ 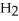 O(l)⇌ N
O(l)⇌ N 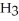 (aq)+
(aq)+ 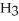
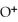 (aq)
(aq)
(Multiple Choice)
4.9/5  (31)
(31)
Showing 41 - 60 of 67
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)