Exam 1: Matter, measurements, and Calculations
Exam 1: Matter, measurements, and Calculations92 Questions
Exam 2: Atoms and Molecules89 Questions
Exam 3: Electronic Structure and the Periodic Law86 Questions
Exam 4: Forces Between Particles89 Questions
Exam 5: Chemical Reactions88 Questions
Exam 6: The States of Matter88 Questions
Exam 7: Solutions and Colloids87 Questions
Exam 8: Reaction Rates and Equilibrium86 Questions
Exam 9: Acids, bases, and Salts86 Questions
Exam 10: Radioactivity and Nuclear Processes86 Questions
Exam 11: Organic Compounds: Alkanes87 Questions
Exam 12: Unsaturated Hydrocarbons88 Questions
Exam 13: Alcohols, phenols, and Ethers86 Questions
Exam 14: Aldehydes and Ketones86 Questions
Exam 15: Carboxylic Acids and Esters86 Questions
Exam 16: Amines and Amides85 Questions
Exam 17: Carbohydrates88 Questions
Exam 18: Lipids91 Questions
Exam 19: Proteins89 Questions
Exam 20: Enzymes88 Questions
Exam 21: Nucleic Acids and Protein Synthesis90 Questions
Exam 22: Nutrition and Energy for Life89 Questions
Exam 23: Carbohydrate Metabolism90 Questions
Exam 24: Lipid and Amino Acid Metabolism94 Questions
Exam 25: Body Fluids86 Questions
Select questions type
Do the following calculation and express the answer using the correct number of significant figures.______ = (342)* (0.0012)÷ 100.0
(Multiple Choice)
4.8/5  (39)
(39)
A particular medication is a heterogeneous mixture.Since heterogeneous mixtures are consistent throughout,this medication does not need to be shaken.
(True/False)
4.9/5  (30)
(30)
If the first ingredient on the label of a cosmetic is "active ingredient",the cosmetic is also regulated as a drug.
(True/False)
4.8/5  (40)
(40)
How can the volume of an irregular unknown object be measured?
(Multiple Choice)
4.8/5  (38)
(38)
If 3333 is divided by 5.0,the answer should have two significant figures.
(True/False)
4.7/5  (41)
(41)
Two pure substances A and B react to form a new pure substance C.From this,we may conclude that
(Multiple Choice)
4.7/5  (39)
(39)
How many significant figures are used in expressing a measurement as 0.2503 L?
(Multiple Choice)
4.8/5  (35)
(35)
Molarity (M)is calculated as: 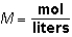 .M would be considered a derived unit.
.M would be considered a derived unit.
(True/False)
4.8/5  (42)
(42)
A sample of urine is measured to have the density of 1.15 g/mL which is an indicator that there may be a medical problem.
(True/False)
4.8/5  (42)
(42)
Which of the following is an example of a homogeneous mixture?
(Multiple Choice)
4.8/5  (33)
(33)
As two clear liquid solutions are thoroughly mixed,a red solid forms.This change is most likely _____ .
(Multiple Choice)
4.9/5  (37)
(37)
After heating,a pure substance,A,is found to produce both B and C.What can be said about the substance A?
(Multiple Choice)
4.8/5  (38)
(38)
Express the following "generic" number in standard notation. X.XX *104
(Multiple Choice)
4.9/5  (36)
(36)
It turns out that the dark side of the moon has as a mean temperature of -280 oF.What would that the temperature be on the Kelvin scale?
(Multiple Choice)
4.8/5  (35)
(35)
If a 200 gram sample of water is partially frozen forming 40 g of ice,than 80% of the original sample is still a liquid.
(True/False)
4.8/5  (38)
(38)
Over the counter (OTC)medicines are dangerous because they are not regulated.
(True/False)
4.8/5  (34)
(34)
Showing 61 - 80 of 92
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)