Exam 2: Measurements and Calculations
Exam 1: Chemistry: an Introduction4 Questions
Exam 2: Measurements and Calculations121 Questions
Exam 3: Matter55 Questions
Exam 4: Chemical Foundations: Elements, Atoms, and Ions101 Questions
Exam 5: Nomenclature219 Questions
Exam 6: Chemical Reactions: an Introduction76 Questions
Exam 7: Reactions in Aqueous Solutions64 Questions
Exam 8: Chemical Composition120 Questions
Exam 9: Chemical Quantities84 Questions
Exam 10: Energy55 Questions
Exam 11: Modern Atomic Theory99 Questions
Exam 12: Chemical Bonding110 Questions
Exam 13: Gases83 Questions
Exam 14: Liquids and Solids39 Questions
Exam 15: Solutions70 Questions
Exam 16: Acids and Bases49 Questions
Exam 17: Equilibrium56 Questions
Exam 18: Oxidation-Reduction Reactions and Electrochemistry81 Questions
Exam 19: Radioactivity and Nuclear Energy26 Questions
Exam 20: Organic Chemistry58 Questions
Exam 21: Biochemistry22 Questions
Select questions type
The product of 0.1400 * 6.02 *1023 will have how many significant figures?
(Multiple Choice)
4.8/5  (36)
(36)
What volume would be occupied by a piece of aluminum (density = 2.70 g/mL) weighing 98.0 g?
(Multiple Choice)
4.8/5  (34)
(34)
Using zero as your reference point, how much liquid has left the buret? Use the correct number of significant figures. 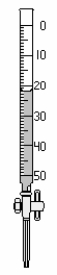
(Multiple Choice)
4.9/5  (41)
(41)
A solid object with a volume of 5.62 mL weighs 108
g. Would this object float or sink in mercury? Explain. (Density of Hg = 13.6 g/mL.)
(Essay)
4.8/5  (34)
(34)
Aluminum has a density of 2.70 g/cm3. What is the mass of a rectangular block of aluminum measuring 11.1 cm by 22.2 cm by 34.5 cm?
(Multiple Choice)
4.8/5  (30)
(30)
A chunk of sulfur has a volume of 5.95 cm3. What is the mass of this sulfur? (Density of sulfur = 2.07 g/cm3.)
(Multiple Choice)
4.8/5  (35)
(35)
An object has a mass of 40.1 g and occupies a volume of 7.67 mL. The density of this object is
(Multiple Choice)
4.8/5  (36)
(36)
How many significant figures are there in the result of the following calculation? (4.321/2.8)* (6.9234 * 105)
(Multiple Choice)
4.8/5  (34)
(34)
Cesium melts at 302 K and boils at 944 K. What would be the physical state of cesium at 25°C?
(Short Answer)
4.8/5  (37)
(37)
If a 100.-g sample of a metal has a volume of 8.65 mL, what is the density of the metal?
(Multiple Choice)
4.8/5  (29)
(29)
A piece of an unknown metal weighs 400.1 g and occupies a volume of 72.2 mL. What is the density of this metal?
(Multiple Choice)
4.8/5  (40)
(40)
How many significant figures should there be in the answer when you divide 4.1 by 7.464?
(Multiple Choice)
4.8/5  (43)
(43)
Showing 41 - 60 of 121
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)