Exam 2: Measurements and Calculations
Exam 1: Chemistry: an Introduction4 Questions
Exam 2: Measurements and Calculations121 Questions
Exam 3: Matter55 Questions
Exam 4: Chemical Foundations: Elements, Atoms, and Ions101 Questions
Exam 5: Nomenclature219 Questions
Exam 6: Chemical Reactions: an Introduction76 Questions
Exam 7: Reactions in Aqueous Solutions64 Questions
Exam 8: Chemical Composition120 Questions
Exam 9: Chemical Quantities84 Questions
Exam 10: Energy55 Questions
Exam 11: Modern Atomic Theory99 Questions
Exam 12: Chemical Bonding110 Questions
Exam 13: Gases83 Questions
Exam 14: Liquids and Solids39 Questions
Exam 15: Solutions70 Questions
Exam 16: Acids and Bases49 Questions
Exam 17: Equilibrium56 Questions
Exam 18: Oxidation-Reduction Reactions and Electrochemistry81 Questions
Exam 19: Radioactivity and Nuclear Energy26 Questions
Exam 20: Organic Chemistry58 Questions
Exam 21: Biochemistry22 Questions
Select questions type
Perform the following conversion: 5.39 m/s = __________ km/h
(Multiple Choice)
4.9/5  (36)
(36)
Calculate the mass of a rectangular solid that has a density of 3.96 g/cm3 and measures 2.50 cm by 1.80 cm by 3.00 cm.
(Multiple Choice)
4.8/5  (34)
(34)
A toy measures 39.1 cm in length. How many inches does this represent?
(Multiple Choice)
4.8/5  (31)
(31)
Perform the following conversion: 6.41 m/s = __________ mi/h
(Multiple Choice)
4.7/5  (43)
(43)
How many significant figures are in the number 0.02020 * 1015?
(Multiple Choice)
5.0/5  (37)
(37)
You take 20.0 mL of water from a graduated cylinder and add it to the beaker of water below. What is the new volume of water in the beaker?
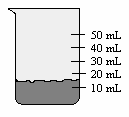
(Multiple Choice)
4.9/5  (32)
(32)
How many significant figures are in the number 60.02 * 105?
(Multiple Choice)
4.7/5  (32)
(32)
The result of the following calculation has how many significant figures? (0.4333 J/g °C) (33.12°C - 31.12°C)(412.1 g)
(Multiple Choice)
4.8/5  (43)
(43)
Water has a density of 1.0 g/mL. Which of these objects will float in water? Object I: mass = 50.0 g; volume = 69.4 mL
Object II: mass = 60.9 g; volume = 54.7 mL
Object III: mass = 100.0 g; volume = 40.0 mL
(Multiple Choice)
4.8/5  (34)
(34)
Showing 81 - 100 of 121
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)