Exam 5: Gases, Liquids, and Solids
Exam 1: Matter, Energy, and Measurement143 Questions
Exam 2: Atoms133 Questions
Exam 3: Chemical Bonds140 Questions
Exam 4: Chemical Reactions140 Questions
Exam 5: Gases, Liquids, and Solids132 Questions
Exam 6: Solutions and Colloids153 Questions
Exam 7: Reaction Rates and Chemical Equilibrium103 Questions
Exam 8: Acids and Bases197 Questions
Exam 9: Nuclear Chemistry155 Questions
Exam 10: Organic Chemistry70 Questions
Exam 11: Alkanes142 Questions
Exam 12: Alkenes and Alkynes123 Questions
Exam 13: Benzene and Its Derivatives60 Questions
Exam 14: Alcohols, Ethers, and Thiols122 Questions
Exam 15: Chirality: the Handedness of Molecules92 Questions
Exam 16: Amines89 Questions
Exam 17: Aldehydes and Ketones101 Questions
Exam 18: Carboxylic Acids115 Questions
Exam 19: Carboxylic Anhydrides, Esters, and Amides117 Questions
Exam 20: Carbohydrates103 Questions
Exam 21: Lipids132 Questions
Exam 22: Proteins128 Questions
Exam 23: Enzymes62 Questions
Exam 24: Chemical Communications: Neurotransmitters and Hormones89 Questions
Exam 25: Nucleotides, Nucleic Acids, and Heredity121 Questions
Exam 26: Gene Expression and Protein Synthesis129 Questions
Exam 27: Bioenergetics: How the Body Converts Food to Energy140 Questions
Exam 28: Specific Catabolic Pathways: Carbohydrate, Lipid, and Protein Metabolism104 Questions
Exam 29: Biosynthetic Pathways67 Questions
Exam 30: Nutrition73 Questions
Exam 31: Immunochemistry142 Questions
Exam 32: Body Fluids72 Questions
Select questions type
Consider a substance as represented by the following models. 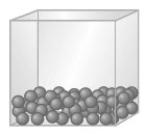

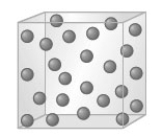 A B C When sugar is heated it forms a mass called caramel. Based on this information sugar does not normally exist as shown in which model?
A B C When sugar is heated it forms a mass called caramel. Based on this information sugar does not normally exist as shown in which model?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following molecules can engage in hydrogen bonding?
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following molecules can have dipole-dipole interactions?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following increases as temperature increases?
(Multiple Choice)
4.8/5  (41)
(41)
A sample of carbon dioxide occupies 22.4 L at STP. Which of the following statements apply to the sample?
(Multiple Choice)
4.7/5  (25)
(25)
What temperature scale should be used if we wish to express Charles's Law as a simple direct proportionality?
(Multiple Choice)
4.9/5  (43)
(43)
Chloroform has a normal boiling point of 61.7°C. Which of the following is true?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following molecules cannot engage in hydrogen bonding?
(Multiple Choice)
4.7/5  (45)
(45)
Which of the following is a mathematical statement of Charles's Law?
(Multiple Choice)
4.8/5  (35)
(35)
Dry air is 78.08% nitrogen, 20.95% oxygen and 0.93% argon with the 0.04% being other gases. If the atmospheric pressure is 760.0 torr, what is the partial pressure of nitrogen in dry air?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following is necessary for a molecule engage in hydrogen bonding?
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following is a mathematical statement of Boyle's Law?
(Multiple Choice)
4.7/5  (30)
(30)
At constant temperature the pressure on a 6.0 L sample of a gas is reduced from 2.0 atm to 1.0 atm. What is the new volume of the gas sample?
(Multiple Choice)
4.8/5  (44)
(44)
Which gas law is most directly related to the process of inhaling and exhaling?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following will occur if the temperature of a gas is increased from 20 K to 40 K at constant pressure?
(Multiple Choice)
4.9/5  (26)
(26)
Which of the following molecules can have only London dispersion forces?
(Multiple Choice)
4.7/5  (33)
(33)
Which law relates the volume and temperature of a gas under conditions of constant pressure?
(Multiple Choice)
4.9/5  (28)
(28)
Which of the following molecules cannot engage in hydrogen bonding?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following could be a correct set of units for the ideal gas law constant, R?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following might correspond to the O2 pressure in a hyperbaric chamber?
(Multiple Choice)
4.9/5  (39)
(39)
Showing 101 - 120 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)