Exam 5: Gases, Liquids, and Solids
Exam 1: Matter, Energy, and Measurement143 Questions
Exam 2: Atoms133 Questions
Exam 3: Chemical Bonds140 Questions
Exam 4: Chemical Reactions140 Questions
Exam 5: Gases, Liquids, and Solids132 Questions
Exam 6: Solutions and Colloids153 Questions
Exam 7: Reaction Rates and Chemical Equilibrium103 Questions
Exam 8: Acids and Bases197 Questions
Exam 9: Nuclear Chemistry155 Questions
Exam 10: Organic Chemistry70 Questions
Exam 11: Alkanes142 Questions
Exam 12: Alkenes and Alkynes123 Questions
Exam 13: Benzene and Its Derivatives60 Questions
Exam 14: Alcohols, Ethers, and Thiols122 Questions
Exam 15: Chirality: the Handedness of Molecules92 Questions
Exam 16: Amines89 Questions
Exam 17: Aldehydes and Ketones101 Questions
Exam 18: Carboxylic Acids115 Questions
Exam 19: Carboxylic Anhydrides, Esters, and Amides117 Questions
Exam 20: Carbohydrates103 Questions
Exam 21: Lipids132 Questions
Exam 22: Proteins128 Questions
Exam 23: Enzymes62 Questions
Exam 24: Chemical Communications: Neurotransmitters and Hormones89 Questions
Exam 25: Nucleotides, Nucleic Acids, and Heredity121 Questions
Exam 26: Gene Expression and Protein Synthesis129 Questions
Exam 27: Bioenergetics: How the Body Converts Food to Energy140 Questions
Exam 28: Specific Catabolic Pathways: Carbohydrate, Lipid, and Protein Metabolism104 Questions
Exam 29: Biosynthetic Pathways67 Questions
Exam 30: Nutrition73 Questions
Exam 31: Immunochemistry142 Questions
Exam 32: Body Fluids72 Questions
Select questions type
At constant temperature the pressure on a 10.0 L sample of gas is changed from 1.00 atm to 1140 torr. What is the new volume of the gas sample?
(Multiple Choice)
5.0/5  (29)
(29)
Which of the following will occur if the temperature of a gas is decreased from 60 K to 30 K at constant pressure?
(Multiple Choice)
4.9/5  (44)
(44)
According to the kinetic molecular theory, which of the following is not true?
(Multiple Choice)
4.8/5  (35)
(35)
A vessel under 2.015 atm pressure contains nitrogen, N2, and water vapor, H2O. The partial pressure of N2 is 1.908 atm. What is the partial pressure of the water vapor?
(Multiple Choice)
5.0/5  (38)
(38)
Consider the following heating curve for the generic substance Z. 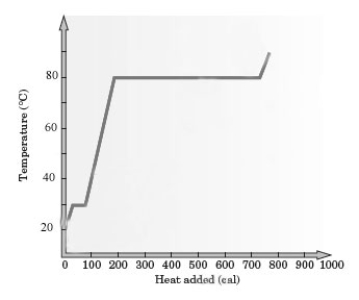 -About how much energy was added to convert all of this sample of Z from the liquid at the boiling point to the gas at the boiling point?
-About how much energy was added to convert all of this sample of Z from the liquid at the boiling point to the gas at the boiling point?
(Multiple Choice)
4.8/5  (33)
(33)
The normal boiling point of acetic acid is 117.9°C. Which of the following is true?
(Multiple Choice)
4.9/5  (47)
(47)
Consider the following phase diagram of water. The temperature and pressure scales are greatly reduced (and are non-linear) 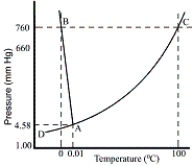 -Crossing the curve designated AD in the direction of high temperature to low temperature is associated with which of the following phase transitions?
-Crossing the curve designated AD in the direction of high temperature to low temperature is associated with which of the following phase transitions?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following molecules can have dipole-dipole interactions?
(Multiple Choice)
4.9/5  (35)
(35)
A sample of carbon dioxide occupies 22.4 L at STP. Which of the following statements apply to the sample?
(Multiple Choice)
4.8/5  (32)
(32)
If 2.00 moles of NO gas occupies 10.0 L at 295 K, what is the pressure of the gas in atmospheres?
(Multiple Choice)
4.8/5  (36)
(36)
Consider the following phase diagram of water. The temperature and pressure scales are greatly reduced (and are non-linear)  -Point A is associated with which of the following?
-Point A is associated with which of the following?
(Multiple Choice)
4.8/5  (32)
(32)
Standard temperature and pressure are which of the following?
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following have an effect on the strength of London dispersion forces?
(Multiple Choice)
4.8/5  (43)
(43)
At constant temperature the pressure on a 6.0 L sample of gas is increased from 1.0 atm to 2.0 atm. What is the new volume of the gas sample?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following characteristics of a liquid sample is associated with the intermolecular forces which are present?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following instruments is used to measure atmospheric pressure?
(Multiple Choice)
4.8/5  (40)
(40)
Consider the following heating curve for the generic substance Z.  -What is the melting point of substance Z?
-What is the melting point of substance Z?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following is a mathematical statement of Gay-Lussac's Law?
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following is true of London dispersion forces?
(Multiple Choice)
4.8/5  (29)
(29)
Consider the following phase diagram of water. The temperature and pressure scales are greatly reduced (and are non-linear)  -Crossing the line designated AB in the direction of high pressure to low pressure is associated with which of the following phase transitions?
-Crossing the line designated AB in the direction of high pressure to low pressure is associated with which of the following phase transitions?
(Multiple Choice)
4.9/5  (30)
(30)
Showing 41 - 60 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)