Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids
Exam 1: Chemistry: the Central Science129 Questions
Exam 2: Atoms Molecules and Ions120 Questions
Exam 3: Stoichiometry Ratios of Combination131 Questions
Exam 4: Reactions in Aqueous Solutions144 Questions
Exam 5: Thermochemistry134 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms107 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I Basic Concepts95 Questions
Exam 9: Chemical Bonding Ii Molecular Geometry and Bonding Theories137 Questions
Exam 10: Gases131 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids135 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions147 Questions
Exam 14: Chemical Kinetics114 Questions
Exam 15: Chemical Equilibrium135 Questions
Exam 16: Acids and Bases133 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria129 Questions
Exam 18: Entropy Free Energy and Equilibrium61 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry125 Questions
Exam 21: Environmental Chemistry131 Questions
Exam 22: Coordination Chemistry128 Questions
Exam 23: Metallurgy and the Chemistry of Metals115 Questions
Exam 24: Nonmetallic Elements and Their Compounds119 Questions
Exam 25: Organic Chemistry148 Questions
Select questions type
Below is the phase diagram for sulfur.Which phase is present at 100°C and 1 atm? 
(Multiple Choice)
4.7/5  (37)
(37)
What is the intermolecular force that exists between a calcium ion and water?
(Multiple Choice)
4.8/5  (35)
(35)
Iron crystallizes in the body-centered cubic lattice.What is the coordination number for Fe?
(Multiple Choice)
4.9/5  (38)
(38)
______________ is the name given to the phase change from a gas directly to a liquid.
(Short Answer)
4.8/5  (48)
(48)
What is the energy in kJ/mol required to melt 1 mole of a solid?
(Multiple Choice)
4.8/5  (33)
(33)
Liquid ammonia can be used as a refrigerant and heat transfer fluid.How much energy is required to heat 25.0 g of NH3(l)from -65.0°C to -12.0°C? Normal boiling point -33.4°C
Specific heat of NH3(l)4.7 J/g • °C
Specific heat of NH3(g)2.2 J/g • °C
Molar heat of vaporization 23.5 kJ/mol
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following properties indicates the presence of strong intermolecular forces in a liquid?
(Multiple Choice)
4.9/5  (41)
(41)
What mass of water would need to evaporate from your skin in order to dissipate 1.70 ×105 J of heat from the surface of your body? H2O(l)→ H2O(g)ΔHvap = 40.7 kJ/mol
(Multiple Choice)
4.9/5  (44)
(44)
Which substance should exhibit hydrogen bonding in the liquid phase?
(Multiple Choice)
4.9/5  (43)
(43)
What name is given to the curved surface of a liquid contained in a narrow tube?
(Multiple Choice)
4.8/5  (28)
(28)
Select the pair of substances in which the one with the lower vapor pressure at a given temperature is listed first.
(Multiple Choice)
4.8/5  (34)
(34)
The surface tension of water is lowered when a detergent is present in solution.
(True/False)
4.8/5  (36)
(36)
Based on the phase diagram of a pure substance given below,which statement is true? 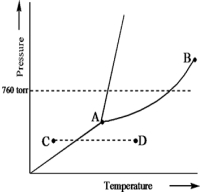
(Multiple Choice)
4.8/5  (33)
(33)
The strongest intermolecular interactions between hydrogen sulfide (H2S)molecules arise from
(Multiple Choice)
4.9/5  (39)
(39)
Based on the phase diagram of a pure substance given below,what phase exists at point F? 
(Multiple Choice)
4.8/5  (41)
(41)
The strongest intermolecular interactions between pentane (C5H12)molecules arise from
(Multiple Choice)
4.9/5  (37)
(37)
What is the attractive force between like molecules involved in capillary action?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 81 - 100 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)